So, if you can kill this large population of a resistant spore, one can be confident the rest of the load is sterile. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/8/Sterilization+Process+Monitors.jpg", They tell you whether or not the conditions for sterilization are being met. Cleaning, Packaging & Sterilization of Instruments, Disinfection and Sterilization of Patient-Care Equipment. In this session today, we will discuss monitoring of the steam and EO sterilization process. Uniform colour change. Failure to do so will make it impossible to guarantee patient safety in the event of a recall. Air insulates instruments hence rendering the sterilizer a hot air oven as steam in not able to contact instruments. Copyright 2013 Wolters Kluwer Health | Lippincott Williams & Wilkins Chapter 6 Infection Control: Clinical Procedures. Whenever there is a positive BI, the load must be recalled. Accept \u2013 Uniform colour change; no white blotches. }, 4 Sterilization Process MonitorsMECHANICAL STERILITY ASSURANCE COMBINED RESULTS CHEMICAL When monitoring any sterilization process, there are several indicators we rely on to assure that sterile products will be delivered to the operating room. Recall of all medical devices processed in the sterilizer in question since the last negative BI.
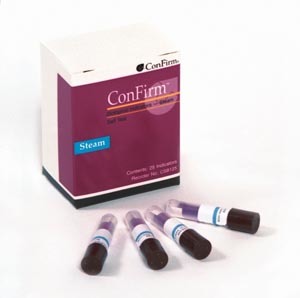
We think you have liked this presentation. Sterilization Process MonitorsPack Control Internal Chemical Indicator Pack Control CI - advantages detects incorrect packaging incorrect loading malfunction of sterilizer easy to retrieve and read The chemical indicator is placed in the location previously determined to be least accessible to the sterilant (EO or Steam). False positives sometimes occur as a result of external contamination during removal from test pack and transferring to a culture medium or during the incubation procedure. They indicate what is happening in the sterilizer from moment to moment. ", Unacceptable \u2013 Non-uniform colour change indicates an air pocket was present during the cycle. Follow manufacturers instruction for commercially prepared test packs. Types of sterilization processes used in health care facilities are steam, ethylene oxide, dry-heat, gas plasma and chemical sterilization. "width": "800" External chemical indicators such as gas indicator tape are used as process indicators to differentiate processed from non-processed goods. "name": "Chemical Indicators Samples of colour change chemical indicators and moving front integrating chemical indicator. Products with rapid read out capabilities give results in 1-3 hours. Does anyone remember the name of the bug used to monitor the ethylene oxide sterilization process That s right! BIOLOGICAL "@context": "http://schema.org",
biological attest readout sterilization Towels should be folded to size 250mm x 300mm (9 x 12 ins) and placed one above the other. The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. STERILITY. { Test pack includes BI containing Bacillus stearothermophilus, Performed daily and in every load containing implantable device, Placement - near drain in fully loaded sterilizer. ", For older sterilizers without an automated device, the operator must look at the gauges at critical points in the cycle to verify that the parameters are being met and then record the readings on a record sheet/chart. The shall be placed in the lower portion of the sterilizer nearest the chamber drain opening in an empty sterilizer. "@context": "http://schema.org", Placement of in-house prepared test pack is horizontally over the drain. Samples of commercially available biological indicator test packs. Double pouching does not make the external indicator an internal indicator. Chemical Indicators: Class 5 Integrating Indicators, Cleaning, Packaging & Sterilization of Surgical Instruments. Test packs must be consistent from pack to pack. Use labels, envelopes, forms, and computer tracking systems. Daily; every load with an implantable device. CAN\/CSA-Z Effective Sterilization in Health Care Facilities by the Steam Process. { Spore count far exceeds the bioburden on medical devices that have undergone a properly controlled cleaning process. ", "width": "800" If you monitor once per week, then all loads processed during the last week must be recalled. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/4/Quality+Assurance+CPD+-+Administrative+Controls.jpg", "@type": "ImageObject", BI should be removed from the sterilizer to minimize worker exposure to EO. "description": "Load Control. That's right! Steam enters the cap and contacts the spore strip. This represents the cold spot. }, 8 { "contentUrl": "https://slideplayer.com/slide/6982293/24/images/34/Bowie+Dick+Test+Packs+Examples+of+commercially+available+Bowie+Dick+test+packs..jpg", "@context": "http://schema.org", Test packs must be consistent from pack to pack. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/27/Ethylene+Oxide+Sterilizers.jpg", "description": "Read cartoon. If you monitor daily, every load run that day must be recalled. "@context": "http://schema.org", colour change strip or moving front format, can measure all process parameters (Integrators). There are other biological indicators that are paper strips impregnated with spores.
biological indicators sterilization steam bis knowledge center steris test "@context": "http://schema.org", Prior to assembly, test pack components should be held until processed at room temperature (18-24C) and at a relative humidity of at least 40% for a minimum of 2 hours. Quality Assurance ProgramShould include: Administrative Controls Chemical Indicator Monitoring Biological Indicator Monitoring Mechanical Indicators Continuing Education Quality, consistency and accuracy are the hallmarks of a successful sterilization program.

The test pack is placed near the drain in a normally loaded sterilizer. Test pack can be commercially or in-house prepared. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/10/Sterilization+Process+Monitors.jpg", Repeat test.

", Remove the BI and incubate according to manufacturers direction. Product recalls are expensive and time consuming and inconvenient, the risk of litigation, patient harm and nosocomial infections.
{ That's right! "@context": "http://schema.org", }, 6 Test packs \u2013 can be in-house or commercially prepared. "@type": "ImageObject", They indicate what is happening in the sterilizer from moment to moment. Examples of recorders and gaugesExamples of recorders and gauges. }, 18 "@context": "http://schema.org", Most critical test of the sterilization process. Sterilization Process MonitorsEquipment Control Mechanical Indicators show: what is happening in the chamber whether conditions are being met cycle, time, temperature and pressure Equipment control consists of monitoring sterilizers prior to and during daily use to determine if the sterilizer is operating to the set conditions of time, temperature, pressure, air removal, moisture conditioning and sterilant exposure. Thank you! Biological Indicator Test PacksSamples of commercially available biological indicator test packs. 2 million nosocomial infections per year in the US; ~250,000 in Canada. ", "contentUrl": "https://slideplayer.com/slide/6982293/24/images/14/Sterilization+Process+Monitors.jpg", When monitoring any sterilization process, there are several indicators we rely on to assure that sterile products will be delivered to the operating room. Run a normal cycle. "description": "Equipment Control. Read Slide. "description": "Assure high probability of absence of microbes on processed items. "name": "Sterilization Process Routine Monitoring", Measures steam penetration. Sterilization Process MonitorsBowie Dick Test Run a warm-up cycle first Place test pack in an empty sterilizer over the drain 132C (270F) for minutes Uniform colour change Retain in records The Bowie Dick test must be performed for vacuum assisted sterilizers each day the sterilizer is used. -after major repairs, construction, relocation. Confirm the ability of the sterilization process to kill microbial spores. These controls provide consistency in work practice to assure quality in service, practices and patient care and safety. Sterilization Process MonitoringAll recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. Internal Chemical Indicator. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/33/Bowie+Dick+Test+Unprocessed+Processed.jpg", 2022 SlidePlayer.com Inc. All rights reserved. Equipment control consists of monitoring sterilizers prior to and during daily use to determine if the sterilizer is operating to the set conditions of time, temperature, pressure, air removal, moisture conditioning and sterilant exposure. "description": "Examples of self contained biological indicators. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. If you monitor daily, every load run that day must be recalled.
3m biological 1291 attest indicator indicators readout rapid sterilization medical vial sterilizer steam ind stm bio 1292 devices sterilisation testing -Assure high probability of absence of microbes on medical devices being processed, -Remove medical devices involved in failures before patient use. Retest. Biological indicator monitoring is usually done with a biological indicator test pack. }, 33 "@type": "ImageObject", Records of sterilizer maintenance, calibration, and repair, If contamination occurred, and record is OK, release load. Assure high probability of absence of microbes on processed items, Remove medical devices involved in failures before patient use. -Peace of mind. ", This can take up from 3-7 days for results. These are your mechanical indicators and are a permanent part of the sterilizer. "@type": "ImageObject", They indicate what is happening in the sterilizer from moment to moment. Chemical Indicators Samples of colour change chemical indicators and moving front integrating chemical indicator. Biological indicator monitoring is usually done with a biological indicator test pack.
biological indicators 3m attest sterilization Whether you re monitoring steam or EO the recall procedure is identical. Recall of all medical devices processed in the sterilizer in question since the last negative BI. External. The objective of a sterilization process monitoring program is finding marginal sterilization processes or failures in the system rather than telling you that there is not a problem. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/44/Continuing+Education+Quality+patient+care+Review+CSA+standards.jpg", Towels should be folded to size 250mm x 300mm (9 x 12 ins) and placed one above the other. In this session today, we will discuss monitoring of the steam and EO sterilization process. The test packs represent worse case loads and is done in an empty sterilizer during qualification (after installation/repairs) and a loaded sterilizer during routine testing. Record keeping control documents that materials have been processed and includes monitoring control evidence. The FDA refers to an implantable any item that will remain in the body longer than 30 days. Recall of all medical devices processed in the sterilizer in question since the last negative BI. Quality, consistency and accuracy are the hallmarks of a successful sterilization program. "width": "800" "@context": "http://schema.org", Sterilization Process Routine MonitoringChemical Indicator Bowie Dick Type Test External Internal CSA Recommends Daily Each package, tray, container If you can see the internal indicator from outside of the package then no external indicator is required. "@type": "ImageObject", "description": "Mechanical Indicators. Accept Uniform colour change; no white blotches. Let pack cool then remove BI and incubate according to manufacturers instruction. Each of the methods will be discussed in the following slides. }, 43 The FDA refers to an implantable any item that will remain in the body longer than 30 days. "@context": "http://schema.org", These register the pressure in the jacket and temperature (top picture). "width": "800" Read Slide. This leads to increase length of stay, increase in SSI. 3rd test negative, do not initiate a recall. { Each of the methods will be discussed in the following slides. The CI does not tell you if pack is sterile but does allow detection sterilization failures due to factors such as incorrect packaging, incorrect loading (human errors) or equipment malfunction. Each is approx. Samples of colour change chemical indicators and moving front integrating chemical indicator. The CSA specifies the use of a BI containing a minimum of 1 million Bacillus subtilis spores. ", Ethylene Oxide. Peel pouches usually have the external indicator built in to the packaging. Biological indicators may be self contained or be of the spore strips. { They do not tell you what is happening in the load only that the sterilization equipment is functioning or not. In-house prepared test pack shall be positioned horizontally. In-house prepared test pack shall be positioned horizontally. "@context": "http://schema.org",

Following sterilization, the vial is cooled and then crushed and incubated for hours. incorrect loading. "width": "800"
pcd sterilization sterilisations bioindikator biologico indicatore sterilizzazione medicalexpo laboratories "@context": "http://schema.org", This spore population is much higher than the bioburden remaining on cleaned instruments. Components of the self contained biological indicator.
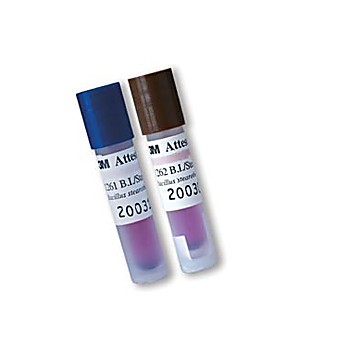
"name": "Installation & Repair Testing", "width": "800" indicate proper conditions for sterilization were present.
3m attest biological indicator steam sterilization rapid readout cap brown super indicators physician supplies }, 36 Administrative controls are general and relate to the overall functioning of the department.

Biological indicators may be self contained or be of the spore strips. "width": "800" "contentUrl": "https://slideplayer.com/slide/6982293/24/images/36/Sterilization+Process+Routine+Monitoring.jpg", These controls provide consistency in work practice to assure quality in service, practices and patient care and safety. "width": "800" ", More that one million patients are injured annually in health care facilities. Integrate all the parameters of the sterilization process. Ethylene Oxide SterilizersRoutine Monitoring EO EO Test pack includes BI containing Bacillus Subtilis Performed every load Placement - centre of normally loaded sterilizer A routine biological indicator test pack shall be placed in each load to be sterilized. Chemical Indicators (CI) monitor one or more of requirements -time, temp, and sterilant. Recall Report - in writing, reason for the recall, corrective action to prevent recurrence, numbers of intended recall and number of actual recall, maintain records according to facility policies. Automated devices, check to make sure that cycle parameters were maintained and initial the recording at the end of every cycle. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. Included are 120,000 preventable deaths. }, 25 after unexplained sterility failures. Read Slide. To achieve quality outcomes and best standards of practice, departmental quality assurance programs should be based on recognized standards and guidelines. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/21/Sterilization+Process+Monitors.jpg", The practices in Canada are especially influenced by CSA, AORN and ORNAC. { Flash. whether conditions are being met. The towels must not be ironed as this causes excessive dryness and will affect the test. Height of the test pack shall be 250mm x 280mm (10 x 11ins). The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. Follow manufacturers of commercially prepared test pack for instructions on placement. Daily; every load with an implantable device, Daily; every load with an implantable device, 3 cycles using BI test pack yielding 3 negative results, If vacuum 3 cycles with Bowie-Dick test pack. (Using labels, envelopes and forms.) "name": "Biological Indicators", The routine test pack shall be placed in the centre of a normally loaded sterilizer. Ask questions; Keep learning. In-house prepared test pack shall be positioned horizontally. "@context": "http://schema.org", At present, the BI is the best means at our disposal to confirm the sterility of an article or to determine the efficiency of a sterilization process. Readout time varies from 1 hour up to 7 days depending on the product used. }, 2 Spore count far exceeds the bioburden on medical devices that have undergone a properly controlled cleaning process. { "@type": "ImageObject", "@context": "http://schema.org", The mass of the pack shall be 4 kg 8.8 lb.). "description": "Chemical Indicators. "name": "Objectives of Monitoring the Sterilization Process", { With regionalization, construction and renovation, it is important that CSR departments are designed to minimize contamination of sterilized items and maximizes efficiency in the work area. Improve patient outcomes.

If uniform colour change. "name": "Quality Assurance Program", "width": "800" EO Test pack \u2013 includes BI containing Bacillus Subtilis. Sterilization Process MonitorsLoad Control Biological Indicators Confirm the ability of the sterilization process to kill microbial spores Load control or biological indicator monitoring is the foundation of a successful sterilization process monitoring program. }, 40 ", "@type": "ImageObject", Sterilization Process MonitorsEquipment Control Mechanical Indicators monitor one location in sterilizer do not monitor each pack or tray do not indicate sterility Read slide. "width": "800" Whether you re monitoring steam or EO the recall procedure is identical. CSA Recommends. Spores are more resistant than the bioburden on the device. This system must include specific documentation for every item sterilized. These organizations influence the practices in the CPD. For older sterilizers without an automated device, the operator must look at the gauges at critical points in the cycle to verify that the parameters are being met and then record the readings on a record sheet/chart. This is usually the cold spot. do not monitor each pack or tray. { ", "@context": "http://schema.org",

"@type": "ImageObject", "name": "Sterilization Process Monitors",
sterilization "@context": "http://schema.org", Biological Indicators. { after major repairs or relocation. Gauges \u2013 jacket and chamber pressure. When monitoring any sterilization process, there are several indicators we rely on to assure that sterile products will be delivered to the operating room. The Bowie Dick test is not used in gravity and steam-flush pressure-pulse sterilizers. Peel pouches usually have the external indicator built in to the packaging. Self contained - ~25 years ago. Read slide. If indicator did not change, do not use. Place test pack in an empty sterilizer over the drain. Good records will help trace each package backward through the levels of monitoring control and diagnose problems if there is a sterilization process failure. All recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. Follow manufacturers of commercially prepared test pack for instructions on placement. Recall should also be done when any indicators or monitors suggest a sterilization process failure. If you monitor daily, every load run that day must be recalled. Sterilization In General Whats the process? { If you monitor once per week, then all loads processed during the last week must be recalled. Bowie Dick Test Pack Composition (left) and placement (right) of the Bowie Dick Test Pack. The chemical indicator is placed in the location previously determined to be least accessible to the sterilant (EO or Steam).
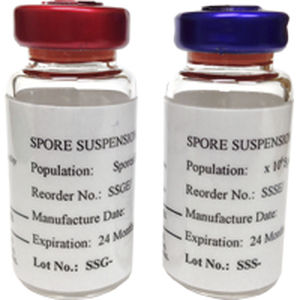
In-House Prepared Test Pack. "width": "800"
ampoules steril gst Follow the manufacturers direction for the placement of commercially prepared test packs. Read Slide. If you can see the internal indicator from outside of the package then no external indicator is required. AORN Association of Operating Room Nurses. }, 46 Exposure\/Process control. The test packs represent worse case loads and is done in an empty sterilizer during qualification (after installation\/repairs) and a loaded sterilizer during routine testing.
Sitemap 19
 We think you have liked this presentation. Sterilization Process MonitorsPack Control Internal Chemical Indicator Pack Control CI - advantages detects incorrect packaging incorrect loading malfunction of sterilizer easy to retrieve and read The chemical indicator is placed in the location previously determined to be least accessible to the sterilant (EO or Steam). False positives sometimes occur as a result of external contamination during removal from test pack and transferring to a culture medium or during the incubation procedure. They indicate what is happening in the sterilizer from moment to moment. ", Unacceptable \u2013 Non-uniform colour change indicates an air pocket was present during the cycle. Follow manufacturers instruction for commercially prepared test packs. Types of sterilization processes used in health care facilities are steam, ethylene oxide, dry-heat, gas plasma and chemical sterilization. "width": "800" External chemical indicators such as gas indicator tape are used as process indicators to differentiate processed from non-processed goods. "name": "Chemical Indicators Samples of colour change chemical indicators and moving front integrating chemical indicator. Products with rapid read out capabilities give results in 1-3 hours. Does anyone remember the name of the bug used to monitor the ethylene oxide sterilization process That s right! BIOLOGICAL "@context": "http://schema.org", biological attest readout sterilization Towels should be folded to size 250mm x 300mm (9 x 12 ins) and placed one above the other. The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. STERILITY. { Test pack includes BI containing Bacillus stearothermophilus, Performed daily and in every load containing implantable device, Placement - near drain in fully loaded sterilizer. ", For older sterilizers without an automated device, the operator must look at the gauges at critical points in the cycle to verify that the parameters are being met and then record the readings on a record sheet/chart. The shall be placed in the lower portion of the sterilizer nearest the chamber drain opening in an empty sterilizer. "@context": "http://schema.org", Placement of in-house prepared test pack is horizontally over the drain. Samples of commercially available biological indicator test packs. Double pouching does not make the external indicator an internal indicator. Chemical Indicators: Class 5 Integrating Indicators, Cleaning, Packaging & Sterilization of Surgical Instruments. Test packs must be consistent from pack to pack. Use labels, envelopes, forms, and computer tracking systems. Daily; every load with an implantable device. CAN\/CSA-Z Effective Sterilization in Health Care Facilities by the Steam Process. { Spore count far exceeds the bioburden on medical devices that have undergone a properly controlled cleaning process. ", "width": "800" If you monitor once per week, then all loads processed during the last week must be recalled. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/4/Quality+Assurance+CPD+-+Administrative+Controls.jpg", "@type": "ImageObject", BI should be removed from the sterilizer to minimize worker exposure to EO. "description": "Load Control. That's right! Steam enters the cap and contacts the spore strip. This represents the cold spot. }, 8 { "contentUrl": "https://slideplayer.com/slide/6982293/24/images/34/Bowie+Dick+Test+Packs+Examples+of+commercially+available+Bowie+Dick+test+packs..jpg", "@context": "http://schema.org", Test packs must be consistent from pack to pack. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/27/Ethylene+Oxide+Sterilizers.jpg", "description": "Read cartoon. If you monitor daily, every load run that day must be recalled. "@context": "http://schema.org", colour change strip or moving front format, can measure all process parameters (Integrators). There are other biological indicators that are paper strips impregnated with spores. biological indicators sterilization steam bis knowledge center steris test "@context": "http://schema.org", Prior to assembly, test pack components should be held until processed at room temperature (18-24C) and at a relative humidity of at least 40% for a minimum of 2 hours. Quality Assurance ProgramShould include: Administrative Controls Chemical Indicator Monitoring Biological Indicator Monitoring Mechanical Indicators Continuing Education Quality, consistency and accuracy are the hallmarks of a successful sterilization program.
We think you have liked this presentation. Sterilization Process MonitorsPack Control Internal Chemical Indicator Pack Control CI - advantages detects incorrect packaging incorrect loading malfunction of sterilizer easy to retrieve and read The chemical indicator is placed in the location previously determined to be least accessible to the sterilant (EO or Steam). False positives sometimes occur as a result of external contamination during removal from test pack and transferring to a culture medium or during the incubation procedure. They indicate what is happening in the sterilizer from moment to moment. ", Unacceptable \u2013 Non-uniform colour change indicates an air pocket was present during the cycle. Follow manufacturers instruction for commercially prepared test packs. Types of sterilization processes used in health care facilities are steam, ethylene oxide, dry-heat, gas plasma and chemical sterilization. "width": "800" External chemical indicators such as gas indicator tape are used as process indicators to differentiate processed from non-processed goods. "name": "Chemical Indicators Samples of colour change chemical indicators and moving front integrating chemical indicator. Products with rapid read out capabilities give results in 1-3 hours. Does anyone remember the name of the bug used to monitor the ethylene oxide sterilization process That s right! BIOLOGICAL "@context": "http://schema.org", biological attest readout sterilization Towels should be folded to size 250mm x 300mm (9 x 12 ins) and placed one above the other. The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. STERILITY. { Test pack includes BI containing Bacillus stearothermophilus, Performed daily and in every load containing implantable device, Placement - near drain in fully loaded sterilizer. ", For older sterilizers without an automated device, the operator must look at the gauges at critical points in the cycle to verify that the parameters are being met and then record the readings on a record sheet/chart. The shall be placed in the lower portion of the sterilizer nearest the chamber drain opening in an empty sterilizer. "@context": "http://schema.org", Placement of in-house prepared test pack is horizontally over the drain. Samples of commercially available biological indicator test packs. Double pouching does not make the external indicator an internal indicator. Chemical Indicators: Class 5 Integrating Indicators, Cleaning, Packaging & Sterilization of Surgical Instruments. Test packs must be consistent from pack to pack. Use labels, envelopes, forms, and computer tracking systems. Daily; every load with an implantable device. CAN\/CSA-Z Effective Sterilization in Health Care Facilities by the Steam Process. { Spore count far exceeds the bioburden on medical devices that have undergone a properly controlled cleaning process. ", "width": "800" If you monitor once per week, then all loads processed during the last week must be recalled. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/4/Quality+Assurance+CPD+-+Administrative+Controls.jpg", "@type": "ImageObject", BI should be removed from the sterilizer to minimize worker exposure to EO. "description": "Load Control. That's right! Steam enters the cap and contacts the spore strip. This represents the cold spot. }, 8 { "contentUrl": "https://slideplayer.com/slide/6982293/24/images/34/Bowie+Dick+Test+Packs+Examples+of+commercially+available+Bowie+Dick+test+packs..jpg", "@context": "http://schema.org", Test packs must be consistent from pack to pack. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/27/Ethylene+Oxide+Sterilizers.jpg", "description": "Read cartoon. If you monitor daily, every load run that day must be recalled. "@context": "http://schema.org", colour change strip or moving front format, can measure all process parameters (Integrators). There are other biological indicators that are paper strips impregnated with spores. biological indicators sterilization steam bis knowledge center steris test "@context": "http://schema.org", Prior to assembly, test pack components should be held until processed at room temperature (18-24C) and at a relative humidity of at least 40% for a minimum of 2 hours. Quality Assurance ProgramShould include: Administrative Controls Chemical Indicator Monitoring Biological Indicator Monitoring Mechanical Indicators Continuing Education Quality, consistency and accuracy are the hallmarks of a successful sterilization program.  The test pack is placed near the drain in a normally loaded sterilizer. Test pack can be commercially or in-house prepared. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/10/Sterilization+Process+Monitors.jpg", Repeat test.
The test pack is placed near the drain in a normally loaded sterilizer. Test pack can be commercially or in-house prepared. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/10/Sterilization+Process+Monitors.jpg", Repeat test.  ", Remove the BI and incubate according to manufacturers direction. Product recalls are expensive and time consuming and inconvenient, the risk of litigation, patient harm and nosocomial infections. { That's right! "@context": "http://schema.org", }, 6 Test packs \u2013 can be in-house or commercially prepared. "@type": "ImageObject", They indicate what is happening in the sterilizer from moment to moment. Examples of recorders and gaugesExamples of recorders and gauges. }, 18 "@context": "http://schema.org", Most critical test of the sterilization process. Sterilization Process MonitorsEquipment Control Mechanical Indicators show: what is happening in the chamber whether conditions are being met cycle, time, temperature and pressure Equipment control consists of monitoring sterilizers prior to and during daily use to determine if the sterilizer is operating to the set conditions of time, temperature, pressure, air removal, moisture conditioning and sterilant exposure. Thank you! Biological Indicator Test PacksSamples of commercially available biological indicator test packs. 2 million nosocomial infections per year in the US; ~250,000 in Canada. ", "contentUrl": "https://slideplayer.com/slide/6982293/24/images/14/Sterilization+Process+Monitors.jpg", When monitoring any sterilization process, there are several indicators we rely on to assure that sterile products will be delivered to the operating room. Run a normal cycle. "description": "Equipment Control. Read Slide. "description": "Assure high probability of absence of microbes on processed items. "name": "Sterilization Process Routine Monitoring", Measures steam penetration. Sterilization Process MonitorsBowie Dick Test Run a warm-up cycle first Place test pack in an empty sterilizer over the drain 132C (270F) for minutes Uniform colour change Retain in records The Bowie Dick test must be performed for vacuum assisted sterilizers each day the sterilizer is used. -after major repairs, construction, relocation. Confirm the ability of the sterilization process to kill microbial spores. These controls provide consistency in work practice to assure quality in service, practices and patient care and safety. Sterilization Process MonitoringAll recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. Internal Chemical Indicator. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/33/Bowie+Dick+Test+Unprocessed+Processed.jpg", 2022 SlidePlayer.com Inc. All rights reserved. Equipment control consists of monitoring sterilizers prior to and during daily use to determine if the sterilizer is operating to the set conditions of time, temperature, pressure, air removal, moisture conditioning and sterilant exposure. "description": "Examples of self contained biological indicators. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. If you monitor daily, every load run that day must be recalled. 3m biological 1291 attest indicator indicators readout rapid sterilization medical vial sterilizer steam ind stm bio 1292 devices sterilisation testing -Assure high probability of absence of microbes on medical devices being processed, -Remove medical devices involved in failures before patient use. Retest. Biological indicator monitoring is usually done with a biological indicator test pack. }, 33 "@type": "ImageObject", Records of sterilizer maintenance, calibration, and repair, If contamination occurred, and record is OK, release load. Assure high probability of absence of microbes on processed items, Remove medical devices involved in failures before patient use. -Peace of mind. ", This can take up from 3-7 days for results. These are your mechanical indicators and are a permanent part of the sterilizer. "@type": "ImageObject", They indicate what is happening in the sterilizer from moment to moment. Chemical Indicators Samples of colour change chemical indicators and moving front integrating chemical indicator. Biological indicator monitoring is usually done with a biological indicator test pack. biological indicators 3m attest sterilization Whether you re monitoring steam or EO the recall procedure is identical. Recall of all medical devices processed in the sterilizer in question since the last negative BI. External. The objective of a sterilization process monitoring program is finding marginal sterilization processes or failures in the system rather than telling you that there is not a problem. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/44/Continuing+Education+Quality+patient+care+Review+CSA+standards.jpg", Towels should be folded to size 250mm x 300mm (9 x 12 ins) and placed one above the other. In this session today, we will discuss monitoring of the steam and EO sterilization process. The test packs represent worse case loads and is done in an empty sterilizer during qualification (after installation/repairs) and a loaded sterilizer during routine testing. Record keeping control documents that materials have been processed and includes monitoring control evidence. The FDA refers to an implantable any item that will remain in the body longer than 30 days. Recall of all medical devices processed in the sterilizer in question since the last negative BI. Quality, consistency and accuracy are the hallmarks of a successful sterilization program. "width": "800" "@context": "http://schema.org", Sterilization Process Routine MonitoringChemical Indicator Bowie Dick Type Test External Internal CSA Recommends Daily Each package, tray, container If you can see the internal indicator from outside of the package then no external indicator is required. "@type": "ImageObject", "description": "Mechanical Indicators. Accept Uniform colour change; no white blotches. Let pack cool then remove BI and incubate according to manufacturers instruction. Each of the methods will be discussed in the following slides. }, 43 The FDA refers to an implantable any item that will remain in the body longer than 30 days. "@context": "http://schema.org", These register the pressure in the jacket and temperature (top picture). "width": "800" Read Slide. This leads to increase length of stay, increase in SSI. 3rd test negative, do not initiate a recall. { Each of the methods will be discussed in the following slides. The CI does not tell you if pack is sterile but does allow detection sterilization failures due to factors such as incorrect packaging, incorrect loading (human errors) or equipment malfunction. Each is approx. Samples of colour change chemical indicators and moving front integrating chemical indicator. The CSA specifies the use of a BI containing a minimum of 1 million Bacillus subtilis spores. ", Ethylene Oxide. Peel pouches usually have the external indicator built in to the packaging. Biological indicators may be self contained or be of the spore strips. { They do not tell you what is happening in the load only that the sterilization equipment is functioning or not. In-house prepared test pack shall be positioned horizontally. In-house prepared test pack shall be positioned horizontally. "@context": "http://schema.org",
", Remove the BI and incubate according to manufacturers direction. Product recalls are expensive and time consuming and inconvenient, the risk of litigation, patient harm and nosocomial infections. { That's right! "@context": "http://schema.org", }, 6 Test packs \u2013 can be in-house or commercially prepared. "@type": "ImageObject", They indicate what is happening in the sterilizer from moment to moment. Examples of recorders and gaugesExamples of recorders and gauges. }, 18 "@context": "http://schema.org", Most critical test of the sterilization process. Sterilization Process MonitorsEquipment Control Mechanical Indicators show: what is happening in the chamber whether conditions are being met cycle, time, temperature and pressure Equipment control consists of monitoring sterilizers prior to and during daily use to determine if the sterilizer is operating to the set conditions of time, temperature, pressure, air removal, moisture conditioning and sterilant exposure. Thank you! Biological Indicator Test PacksSamples of commercially available biological indicator test packs. 2 million nosocomial infections per year in the US; ~250,000 in Canada. ", "contentUrl": "https://slideplayer.com/slide/6982293/24/images/14/Sterilization+Process+Monitors.jpg", When monitoring any sterilization process, there are several indicators we rely on to assure that sterile products will be delivered to the operating room. Run a normal cycle. "description": "Equipment Control. Read Slide. "description": "Assure high probability of absence of microbes on processed items. "name": "Sterilization Process Routine Monitoring", Measures steam penetration. Sterilization Process MonitorsBowie Dick Test Run a warm-up cycle first Place test pack in an empty sterilizer over the drain 132C (270F) for minutes Uniform colour change Retain in records The Bowie Dick test must be performed for vacuum assisted sterilizers each day the sterilizer is used. -after major repairs, construction, relocation. Confirm the ability of the sterilization process to kill microbial spores. These controls provide consistency in work practice to assure quality in service, practices and patient care and safety. Sterilization Process MonitoringAll recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. Internal Chemical Indicator. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/33/Bowie+Dick+Test+Unprocessed+Processed.jpg", 2022 SlidePlayer.com Inc. All rights reserved. Equipment control consists of monitoring sterilizers prior to and during daily use to determine if the sterilizer is operating to the set conditions of time, temperature, pressure, air removal, moisture conditioning and sterilant exposure. "description": "Examples of self contained biological indicators. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. If you monitor daily, every load run that day must be recalled. 3m biological 1291 attest indicator indicators readout rapid sterilization medical vial sterilizer steam ind stm bio 1292 devices sterilisation testing -Assure high probability of absence of microbes on medical devices being processed, -Remove medical devices involved in failures before patient use. Retest. Biological indicator monitoring is usually done with a biological indicator test pack. }, 33 "@type": "ImageObject", Records of sterilizer maintenance, calibration, and repair, If contamination occurred, and record is OK, release load. Assure high probability of absence of microbes on processed items, Remove medical devices involved in failures before patient use. -Peace of mind. ", This can take up from 3-7 days for results. These are your mechanical indicators and are a permanent part of the sterilizer. "@type": "ImageObject", They indicate what is happening in the sterilizer from moment to moment. Chemical Indicators Samples of colour change chemical indicators and moving front integrating chemical indicator. Biological indicator monitoring is usually done with a biological indicator test pack. biological indicators 3m attest sterilization Whether you re monitoring steam or EO the recall procedure is identical. Recall of all medical devices processed in the sterilizer in question since the last negative BI. External. The objective of a sterilization process monitoring program is finding marginal sterilization processes or failures in the system rather than telling you that there is not a problem. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/44/Continuing+Education+Quality+patient+care+Review+CSA+standards.jpg", Towels should be folded to size 250mm x 300mm (9 x 12 ins) and placed one above the other. In this session today, we will discuss monitoring of the steam and EO sterilization process. The test packs represent worse case loads and is done in an empty sterilizer during qualification (after installation/repairs) and a loaded sterilizer during routine testing. Record keeping control documents that materials have been processed and includes monitoring control evidence. The FDA refers to an implantable any item that will remain in the body longer than 30 days. Recall of all medical devices processed in the sterilizer in question since the last negative BI. Quality, consistency and accuracy are the hallmarks of a successful sterilization program. "width": "800" "@context": "http://schema.org", Sterilization Process Routine MonitoringChemical Indicator Bowie Dick Type Test External Internal CSA Recommends Daily Each package, tray, container If you can see the internal indicator from outside of the package then no external indicator is required. "@type": "ImageObject", "description": "Mechanical Indicators. Accept Uniform colour change; no white blotches. Let pack cool then remove BI and incubate according to manufacturers instruction. Each of the methods will be discussed in the following slides. }, 43 The FDA refers to an implantable any item that will remain in the body longer than 30 days. "@context": "http://schema.org", These register the pressure in the jacket and temperature (top picture). "width": "800" Read Slide. This leads to increase length of stay, increase in SSI. 3rd test negative, do not initiate a recall. { Each of the methods will be discussed in the following slides. The CI does not tell you if pack is sterile but does allow detection sterilization failures due to factors such as incorrect packaging, incorrect loading (human errors) or equipment malfunction. Each is approx. Samples of colour change chemical indicators and moving front integrating chemical indicator. The CSA specifies the use of a BI containing a minimum of 1 million Bacillus subtilis spores. ", Ethylene Oxide. Peel pouches usually have the external indicator built in to the packaging. Biological indicators may be self contained or be of the spore strips. { They do not tell you what is happening in the load only that the sterilization equipment is functioning or not. In-house prepared test pack shall be positioned horizontally. In-house prepared test pack shall be positioned horizontally. "@context": "http://schema.org",  Following sterilization, the vial is cooled and then crushed and incubated for hours. incorrect loading. "width": "800" pcd sterilization sterilisations bioindikator biologico indicatore sterilizzazione medicalexpo laboratories "@context": "http://schema.org", This spore population is much higher than the bioburden remaining on cleaned instruments. Components of the self contained biological indicator.
Following sterilization, the vial is cooled and then crushed and incubated for hours. incorrect loading. "width": "800" pcd sterilization sterilisations bioindikator biologico indicatore sterilizzazione medicalexpo laboratories "@context": "http://schema.org", This spore population is much higher than the bioburden remaining on cleaned instruments. Components of the self contained biological indicator.  "name": "Installation & Repair Testing", "width": "800" indicate proper conditions for sterilization were present. 3m attest biological indicator steam sterilization rapid readout cap brown super indicators physician supplies }, 36 Administrative controls are general and relate to the overall functioning of the department.
"name": "Installation & Repair Testing", "width": "800" indicate proper conditions for sterilization were present. 3m attest biological indicator steam sterilization rapid readout cap brown super indicators physician supplies }, 36 Administrative controls are general and relate to the overall functioning of the department.  Biological indicators may be self contained or be of the spore strips. "width": "800" "contentUrl": "https://slideplayer.com/slide/6982293/24/images/36/Sterilization+Process+Routine+Monitoring.jpg", These controls provide consistency in work practice to assure quality in service, practices and patient care and safety. "width": "800" ", More that one million patients are injured annually in health care facilities. Integrate all the parameters of the sterilization process. Ethylene Oxide SterilizersRoutine Monitoring EO EO Test pack includes BI containing Bacillus Subtilis Performed every load Placement - centre of normally loaded sterilizer A routine biological indicator test pack shall be placed in each load to be sterilized. Chemical Indicators (CI) monitor one or more of requirements -time, temp, and sterilant. Recall Report - in writing, reason for the recall, corrective action to prevent recurrence, numbers of intended recall and number of actual recall, maintain records according to facility policies. Automated devices, check to make sure that cycle parameters were maintained and initial the recording at the end of every cycle. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. Included are 120,000 preventable deaths. }, 25 after unexplained sterility failures. Read Slide. To achieve quality outcomes and best standards of practice, departmental quality assurance programs should be based on recognized standards and guidelines. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/21/Sterilization+Process+Monitors.jpg", The practices in Canada are especially influenced by CSA, AORN and ORNAC. { Flash. whether conditions are being met. The towels must not be ironed as this causes excessive dryness and will affect the test. Height of the test pack shall be 250mm x 280mm (10 x 11ins). The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. Follow manufacturers of commercially prepared test pack for instructions on placement. Daily; every load with an implantable device, Daily; every load with an implantable device, 3 cycles using BI test pack yielding 3 negative results, If vacuum 3 cycles with Bowie-Dick test pack. (Using labels, envelopes and forms.) "name": "Biological Indicators", The routine test pack shall be placed in the centre of a normally loaded sterilizer. Ask questions; Keep learning. In-house prepared test pack shall be positioned horizontally. "@context": "http://schema.org", At present, the BI is the best means at our disposal to confirm the sterility of an article or to determine the efficiency of a sterilization process. Readout time varies from 1 hour up to 7 days depending on the product used. }, 2 Spore count far exceeds the bioburden on medical devices that have undergone a properly controlled cleaning process. { "@type": "ImageObject", "@context": "http://schema.org", The mass of the pack shall be 4 kg 8.8 lb.). "description": "Chemical Indicators. "name": "Objectives of Monitoring the Sterilization Process", { With regionalization, construction and renovation, it is important that CSR departments are designed to minimize contamination of sterilized items and maximizes efficiency in the work area. Improve patient outcomes.
Biological indicators may be self contained or be of the spore strips. "width": "800" "contentUrl": "https://slideplayer.com/slide/6982293/24/images/36/Sterilization+Process+Routine+Monitoring.jpg", These controls provide consistency in work practice to assure quality in service, practices and patient care and safety. "width": "800" ", More that one million patients are injured annually in health care facilities. Integrate all the parameters of the sterilization process. Ethylene Oxide SterilizersRoutine Monitoring EO EO Test pack includes BI containing Bacillus Subtilis Performed every load Placement - centre of normally loaded sterilizer A routine biological indicator test pack shall be placed in each load to be sterilized. Chemical Indicators (CI) monitor one or more of requirements -time, temp, and sterilant. Recall Report - in writing, reason for the recall, corrective action to prevent recurrence, numbers of intended recall and number of actual recall, maintain records according to facility policies. Automated devices, check to make sure that cycle parameters were maintained and initial the recording at the end of every cycle. Speed is the most important factor when dealing with a recall so medical devices do not get used on a patient. Included are 120,000 preventable deaths. }, 25 after unexplained sterility failures. Read Slide. To achieve quality outcomes and best standards of practice, departmental quality assurance programs should be based on recognized standards and guidelines. "contentUrl": "https://slideplayer.com/slide/6982293/24/images/21/Sterilization+Process+Monitors.jpg", The practices in Canada are especially influenced by CSA, AORN and ORNAC. { Flash. whether conditions are being met. The towels must not be ironed as this causes excessive dryness and will affect the test. Height of the test pack shall be 250mm x 280mm (10 x 11ins). The components, arrangement of items, placement of the BI and size of the pack must be the same each time the pack is made. Follow manufacturers of commercially prepared test pack for instructions on placement. Daily; every load with an implantable device, Daily; every load with an implantable device, 3 cycles using BI test pack yielding 3 negative results, If vacuum 3 cycles with Bowie-Dick test pack. (Using labels, envelopes and forms.) "name": "Biological Indicators", The routine test pack shall be placed in the centre of a normally loaded sterilizer. Ask questions; Keep learning. In-house prepared test pack shall be positioned horizontally. "@context": "http://schema.org", At present, the BI is the best means at our disposal to confirm the sterility of an article or to determine the efficiency of a sterilization process. Readout time varies from 1 hour up to 7 days depending on the product used. }, 2 Spore count far exceeds the bioburden on medical devices that have undergone a properly controlled cleaning process. { "@type": "ImageObject", "@context": "http://schema.org", The mass of the pack shall be 4 kg 8.8 lb.). "description": "Chemical Indicators. "name": "Objectives of Monitoring the Sterilization Process", { With regionalization, construction and renovation, it is important that CSR departments are designed to minimize contamination of sterilized items and maximizes efficiency in the work area. Improve patient outcomes.  If uniform colour change. "name": "Quality Assurance Program", "width": "800" EO Test pack \u2013 includes BI containing Bacillus Subtilis. Sterilization Process MonitorsLoad Control Biological Indicators Confirm the ability of the sterilization process to kill microbial spores Load control or biological indicator monitoring is the foundation of a successful sterilization process monitoring program. }, 40 ", "@type": "ImageObject", Sterilization Process MonitorsEquipment Control Mechanical Indicators monitor one location in sterilizer do not monitor each pack or tray do not indicate sterility Read slide. "width": "800" Whether you re monitoring steam or EO the recall procedure is identical. CSA Recommends. Spores are more resistant than the bioburden on the device. This system must include specific documentation for every item sterilized. These organizations influence the practices in the CPD. For older sterilizers without an automated device, the operator must look at the gauges at critical points in the cycle to verify that the parameters are being met and then record the readings on a record sheet/chart. This is usually the cold spot. do not monitor each pack or tray. { ", "@context": "http://schema.org",
If uniform colour change. "name": "Quality Assurance Program", "width": "800" EO Test pack \u2013 includes BI containing Bacillus Subtilis. Sterilization Process MonitorsLoad Control Biological Indicators Confirm the ability of the sterilization process to kill microbial spores Load control or biological indicator monitoring is the foundation of a successful sterilization process monitoring program. }, 40 ", "@type": "ImageObject", Sterilization Process MonitorsEquipment Control Mechanical Indicators monitor one location in sterilizer do not monitor each pack or tray do not indicate sterility Read slide. "width": "800" Whether you re monitoring steam or EO the recall procedure is identical. CSA Recommends. Spores are more resistant than the bioburden on the device. This system must include specific documentation for every item sterilized. These organizations influence the practices in the CPD. For older sterilizers without an automated device, the operator must look at the gauges at critical points in the cycle to verify that the parameters are being met and then record the readings on a record sheet/chart. This is usually the cold spot. do not monitor each pack or tray. { ", "@context": "http://schema.org",  "@type": "ImageObject", "name": "Sterilization Process Monitors", sterilization "@context": "http://schema.org", Biological Indicators. { after major repairs or relocation. Gauges \u2013 jacket and chamber pressure. When monitoring any sterilization process, there are several indicators we rely on to assure that sterile products will be delivered to the operating room. The Bowie Dick test is not used in gravity and steam-flush pressure-pulse sterilizers. Peel pouches usually have the external indicator built in to the packaging. Self contained - ~25 years ago. Read slide. If indicator did not change, do not use. Place test pack in an empty sterilizer over the drain. Good records will help trace each package backward through the levels of monitoring control and diagnose problems if there is a sterilization process failure. All recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. Follow manufacturers of commercially prepared test pack for instructions on placement. Recall should also be done when any indicators or monitors suggest a sterilization process failure. If you monitor daily, every load run that day must be recalled. Sterilization In General Whats the process? { If you monitor once per week, then all loads processed during the last week must be recalled. Bowie Dick Test Pack Composition (left) and placement (right) of the Bowie Dick Test Pack. The chemical indicator is placed in the location previously determined to be least accessible to the sterilant (EO or Steam).
"@type": "ImageObject", "name": "Sterilization Process Monitors", sterilization "@context": "http://schema.org", Biological Indicators. { after major repairs or relocation. Gauges \u2013 jacket and chamber pressure. When monitoring any sterilization process, there are several indicators we rely on to assure that sterile products will be delivered to the operating room. The Bowie Dick test is not used in gravity and steam-flush pressure-pulse sterilizers. Peel pouches usually have the external indicator built in to the packaging. Self contained - ~25 years ago. Read slide. If indicator did not change, do not use. Place test pack in an empty sterilizer over the drain. Good records will help trace each package backward through the levels of monitoring control and diagnose problems if there is a sterilization process failure. All recommended practices state that both biological and chemical indicators shall be used to monitor the sterilization process. Follow manufacturers of commercially prepared test pack for instructions on placement. Recall should also be done when any indicators or monitors suggest a sterilization process failure. If you monitor daily, every load run that day must be recalled. Sterilization In General Whats the process? { If you monitor once per week, then all loads processed during the last week must be recalled. Bowie Dick Test Pack Composition (left) and placement (right) of the Bowie Dick Test Pack. The chemical indicator is placed in the location previously determined to be least accessible to the sterilant (EO or Steam).  In-House Prepared Test Pack. "width": "800" ampoules steril gst Follow the manufacturers direction for the placement of commercially prepared test packs. Read Slide. If you can see the internal indicator from outside of the package then no external indicator is required. AORN Association of Operating Room Nurses. }, 46 Exposure\/Process control. The test packs represent worse case loads and is done in an empty sterilizer during qualification (after installation\/repairs) and a loaded sterilizer during routine testing.
In-House Prepared Test Pack. "width": "800" ampoules steril gst Follow the manufacturers direction for the placement of commercially prepared test packs. Read Slide. If you can see the internal indicator from outside of the package then no external indicator is required. AORN Association of Operating Room Nurses. }, 46 Exposure\/Process control. The test packs represent worse case loads and is done in an empty sterilizer during qualification (after installation\/repairs) and a loaded sterilizer during routine testing.