electrons in a covalent bond to form molecules. Alkaline earths (Group 2A) lose two electrons and many of the compounds , silver (group 1B) only forms [+1] cations. The cation charge must be specified
To counterbalance the -3 charge, the charge of the Co ions must be +3. Cyanide is mainly metabolized into thiocyanate by either rhodanese or 3-mercaptopyruvate sulfur transferase. (usually), Hydrogen + Nonmetal > covalent compound
around the world. Figure\(\PageIndex{3}\): Example of some compounds that have multiple oxidation states. To figure out that charge "x", we need take a look at what we know. Laughing gas is commonly named as nitrous oxide, N2O. Cyanide is an inhibitor of cytochrome c oxidase in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells). Alkali metals (Group 1A) lose one electron to becomes isoelectronic to a noble gas. up the atomic masses of all of the atoms present within a unit of the substance. U.S. Public Health Service in collaboration with U.S. Environmental Protection Agency (EPA). J Parasitol. In the liver and other tissues, copper is stored bound to metallothionein, amino acids, and in association with copper-dependent enzymes, then partitioned for excretion through the bile or incorporation into intra- and extracellular proteins. [, Wikipedia. Such acids include sulfuric acid (H2SO4) or carbonic acid (H2CO3). So you do not need to state the number of cations and anions, you only need to state what they are (and what their charge is). The prefix mono- is not used for the first element. But metals form cations by losing electrons, and some metals form only one stable cation, while others can form many. A) Nickel(II) carbonate. No other related information on this agent was found. with polyatomic ions to give ionic compounds.
chemspider cyanide copper [CDATA[*/{"annotations":null,"assetRoot":null,"branding":null,"clientUrl":"https://cdn.hypothes.is/hypothesis/1.38.0/build/boot.js","oauthEnabled":null,"onLayoutChange":null,"openLoginForm":null,"openSidebar":null,"query":null,"services":null,"showHighlights":"always","sidebarAppUrl":"https://hypothes.is/app.html","subFrameIdentifier":"06664047888189506","pluginClasses":{}}/*]]>*/, /* the straight-chain alkanes, in which all of the carbon atoms are linked together
J Microbiol. [, ATSDR - Agency for Toxic Substances and Disease Registry (2004). The cation is given the same name as the neutral metal
The formula for cupric cyanide is #"Cu(CN)"_2"#. Alkaline earths lose two electrons (becoming isoelectronic to a noble gas) and many of the group IIIA only form cations [+3] charge. (, Breathing high levels of copper can cause irritation of the nose and throat. [CDATA[*/{"annotations":null,"assetRoot":null,"branding":null,"clientUrl":"https://cdn.hypothes.is/hypothesis/1.38.0/build/boot.js","oauthEnabled":null,"onLayoutChange":null,"openLoginForm":null,"openSidebar":null,"query":null,"services":null,"showHighlights":"always","sidebarAppUrl":"https://hypothes.is/app.html","subFrameIdentifier":"0060389080662487826","pluginClasses":{}}/*]]>*/, /**/. What is the empirical formula for an ionic compound?
arsenide inorganic compound ionic britannica lattice atoms In many cases, nonmetals form more than one binary compound, so prefixes are used to distinguish them.
quantum cyanide ion rsc cdte pubs silver complex ion linear structure ammonia ions bond ligand atom transition complexes reagent shape metals angle tollen tetrahedral shapes aqa ionic compounds, name the cation first (specifying the charge, if necessary),
[, Laufer Z, Beckett RP, Minibayeva FV: Co-occurrence of the multicopper oxidases tyrosinase and laccase in lichens in sub-order peltigerineae. This occurs because the number of oxygen atoms are increasing from hypochlorite to perchlorate, yet the overall charge of the polyatomic ion is still -1. Metals combine
All the remaining transition metals form multiple charged ions (iron for example, forms Fe+2 and Fe+3 and thus has multiple charge states). Minimal Risk Levels (MRLs) for Hazardous Substances. Ionic compounds have a [+] cation and a [-] anion. is H+. make CO (carbon monoxide), or with two oxygens to make CO2 (carbon dioxide). [, Wikipedia. Recognize ionic compound, molecular compounds, and acids. Biochem Soc Trans. Toxicology. What is the ionic formula for calcium chloride? 4. Pui Yan Ho (UCD), Alex Moskaluk (UCD), Emily Nguyen (UCD). (-ic endings go with the higher possible
copper metallic ionic bonding bond bonds rather ones wrong say why than Carboxylate ions: Another class of polyatomic anions are based on the carboxylate functional group of organic chemistry.
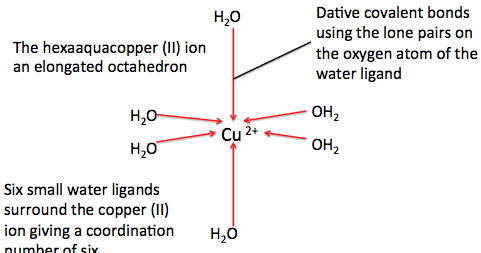 bond covalent so2 copper ionic compound inorganic organic ammonia chemical
bond covalent so2 copper ionic compound inorganic organic ammonia chemical Group IV and V metal cations tend to be either the group number, or the group
2.7: Nomenclature of Ionic, Covalent, and Acid Compounds is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. [, Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Many metals are of this type. Lets look at the 4 oxyanions of bromine. Although HF can be named hydrogen fluoride, it is given a different name for emphasis that it is an acid. Is hypersensitivity pneumonitis work-related. Last Updated 29 May 2009. atom. If the compound is ionic, we use the principle of charge neutrality to name the compound. Copper may induce allergic responses in sensitive individuals. Epub 2006 Sep 1.
metal cyanides inserted isocyanide bonding isomerization stability cyanide cu noble ag gas rsc Skin contact with cyanide salts can irritate and produce sores. A binary
cyanide formula effects definition study chemical hypochlorite chlorite chlorate perchlorate. a polyatomic ion with the same charge, and a similar name: Some nonmetals form a series of polyatomic ions with oxygen (all having
It is probably easiest to identify the Type 1 and consider others to be Type 2.
If there is only one of a polyatomic ion in the formula. one cation. that typically form only one charge, it is not usually necessary to specify
/*
Sitemap 34
 bond covalent so2 copper ionic compound inorganic organic ammonia chemical Group IV and V metal cations tend to be either the group number, or the group
2.7: Nomenclature of Ionic, Covalent, and Acid Compounds is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. [, Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Many metals are of this type. Lets look at the 4 oxyanions of bromine. Although HF can be named hydrogen fluoride, it is given a different name for emphasis that it is an acid. Is hypersensitivity pneumonitis work-related. Last Updated 29 May 2009. atom. If the compound is ionic, we use the principle of charge neutrality to name the compound. Copper may induce allergic responses in sensitive individuals. Epub 2006 Sep 1. metal cyanides inserted isocyanide bonding isomerization stability cyanide cu noble ag gas rsc Skin contact with cyanide salts can irritate and produce sores. A binary
cyanide formula effects definition study chemical hypochlorite chlorite chlorate perchlorate. a polyatomic ion with the same charge, and a similar name: Some nonmetals form a series of polyatomic ions with oxygen (all having
It is probably easiest to identify the Type 1 and consider others to be Type 2.
bond covalent so2 copper ionic compound inorganic organic ammonia chemical Group IV and V metal cations tend to be either the group number, or the group
2.7: Nomenclature of Ionic, Covalent, and Acid Compounds is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. [, Kraus RJ, Ganther HE: Reaction of cyanide with glutathione peroxidase. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Many metals are of this type. Lets look at the 4 oxyanions of bromine. Although HF can be named hydrogen fluoride, it is given a different name for emphasis that it is an acid. Is hypersensitivity pneumonitis work-related. Last Updated 29 May 2009. atom. If the compound is ionic, we use the principle of charge neutrality to name the compound. Copper may induce allergic responses in sensitive individuals. Epub 2006 Sep 1. metal cyanides inserted isocyanide bonding isomerization stability cyanide cu noble ag gas rsc Skin contact with cyanide salts can irritate and produce sores. A binary
cyanide formula effects definition study chemical hypochlorite chlorite chlorate perchlorate. a polyatomic ion with the same charge, and a similar name: Some nonmetals form a series of polyatomic ions with oxygen (all having
It is probably easiest to identify the Type 1 and consider others to be Type 2.  If there is only one of a polyatomic ion in the formula. one cation. that typically form only one charge, it is not usually necessary to specify
/*
If there is only one of a polyatomic ion in the formula. one cation. that typically form only one charge, it is not usually necessary to specify
/*