Analyzing PMS data to compile the required reports, Establishing an EU MDR audit plan that integrates into the current internal auditing plans, Training internal auditor teams on techniques to audit against the MDR, Following up and assist in implementing corrective actions to address nonconformities.
compliance regulatory sketchbubble concept compliance regulatory concept sketchbubble The purpose of the post-market surveillance system is to systematically and proactively collect, record, and analyze data essential for the quality, performance, and safety of the appliance throughout its shelf-life and to update the risk-benefit assessment.
audit medical launcheffecthouston Special caution is recommended for devices intended to administer drugs, since drug-device combination products are regulated under a single set of rules in the US and overseen by a single competent authority whereas they are subject to dual legislation in the EU. For example, demonstrating conformity to the GSPRs, developing PMCF procedures within a PMS system, and performing Clinical Evaluation may all involve the design, documentation, implementation, conduct and interpretation of clinical investigations. The new regulation introduces major changes to how medical device manufacturers obtain CE Marking and maintain access to the European market. Manufacturers will need to demonstrate compliance in order to gain regulatory approval of their medical devices. Devices must be developed within an appropriate Quality Management System (QMS), with most manufacturers applying ISO 13485:2016 as a harmonised standard to ensure QMS suitability. Presented by Steve Greer on June 23, 2020, The first countrywide import alert issued by FDA, An inadequate product specifications and a product recall, A different perspective on process validation and the culpability of the quality unit, How a major pharmaceutical company designed a program to train future leaders in quality, An industry-led initiative to advance the state of quality in the pharma industry, A pharma GMP leaders tips for supporting quality culture within an organization, An update on FDAs Quality Maturity Model, Presented by Paul Smith, Agilent Technologies on Aug. 26, 2020, Presented by Regulatory Compliance Associates Distinguished Fellow Susan Schniepp, The latest U.S. and European regulatory developments, How the EU MDR impacts drug-device combination products. At the most elementary level, EU MDR compliance can be considered in three principal domains: Medical devices must be suitable for their intended purpose, demonstrate a favourable benefit-risk profile and comply with all relevant MDR Annex I General Safety and Performance Requirements (GSPRs). And, the United Kingdom (UK) left the EU in December 2019 and will be setting its own separate process for medical device clearance.

A thorough understanding of the MDR allows you to apply this knowledge, along with guidance from harmonised standards and MedDev guidelines, to begin constructing MDR-compliant systems and processes. But the implementation of stricter regulations through the EU MDR and the tendency to deregulate in the US have changed this picture and invited a lot of criticism that the EU market would lose its attractiveness for medical device innovation. Enter your email address and someone will contact you shortly to customize your insights.
compliance regulatory sketchbubble concept 
info@qaconsultinginc.com, QA Consulting, Inc. All Rights Reserved.Privacy Policy | Terms & Conditions. Identifying root cause for issues identifying and establishing corrective actions to be taken. And the non-standardized UDI systems have finally made it impossible to have the same outer pack label for the US and EU.
compliance regulatory concept sketchbubble template In addition to commercial decision criteria, such as revenue and market share, regulatory requirements are also decisively important. Your regulatory strategy is intertwined with your marketing strategy, as gaining access to markets is preceded by regulatory approval.
strategy sketchbubble WARNING LETTERS Easily search the largest database of FDA Warning Letters by date, company, FDA office, or subject, SITES Quickly assess inspection records including dates and inspectors across all of your sites, INSPECTIONS Complete list of FDA inspections by date, company, category, and country. Medical devices must be designed, manufactured, distributed and tracked according to EU MDR requirements, and manufacturers must develop a suite of compliant regulatory systems, processes and documents to continually monitor the safety and performance of their products.
compliance animation regulatory business concept practice ppt powerpoint template templates presentation slide slides themes presentations poweredtemplate backgrounds google Recently, we published a four-part series of articles that included portions of the Q&A. Get quality and compliance insights from our experts in your inbox. Achieving marketing capital often depends upon a robust and well-built regulatory strategy. MDR Compliance will require a comprehensive clinical evidence portfolio that demonstrates: Meeting these requirements will necessitate: Furthermore, regulatory staff need to have a good working relationship with marketing departments and sales staff, since all marketing and promotional claims made in any marketing material must be backed by appraised clinical evidence. In addition, the various MedDev guidelines provide guidance in structuring and performing a range of MDR Compliance activities. of our MDReady Starter Guide Guide.
Enter your email address and someone will contact you shortly to get the data and analysis you need. Clinical Investigations must minimise the potential for bias, be adequately powered, represent the population normally subject to the device, and employ appropriate methods of statistical analysis in interpreting and applying results. Often, manufacturers utilize their internal team to plan and implement the additional QMS requirements, leaving them without fully independent auditing. Five FDA Warning Letters Issued in Four Years for Fraudulent Cancer Drug, Invest in Tech to Avoid Data Integrity Issues, A comparison of 483 observations in FY2019, Top 10 Russian Ministry of Health inspection findings, Trend analysis of FDA inspections through mid-2020, Strategies for preparing and hosting virtual inspections, New technologies to support remote inspections, How to use the Leadership SOS Model to transform quality culture, How to strengthen quality systems to eliminate human error, How to generate ideas on how to set your organization up for success for shareholders, FDA, and staff, Trends from 2015, 2016, 2018, and 2019 inspections, Conclusions drawn from an analysis of drug inspection data, The 3C Model to become a champion of change, How to identify game-changing habits and the steps to implement them, Ways to develop greater purpose-centered leadership, How to generate awareness along with actions to create changes in how you think about challenges and change putting you on a path to lead change successfully in your organization, Examples of essential laboratory actions to remain compliant during the pandemic, Recent data integrity non-compliance findings and trends, Essential strategies to find, understand, and leverage regulatory non-compliance data, The latest developments regarding the EU MDR, Quality Systems requirements for medical devices, Regulatory updates affecting medical devices, A basic understanding of data sources, machine learning, NLP, and A.I. Performing and interpreting clinical evidence requires a degree of medical insight that many medical device companies do not have available in-house. Despite the deadline, many medical device manufacturers have yet to prepare for compliance with these new requirements or organize their regulatory transition strategies. Successful compliance is demonstrated by the application of a CE-mark to the device. By contrast, most Class I devices do not normally require involvement of a Notified Body but must still be supported by MDR-compliant regulatory files, systems and processes. Meeting the requirements for MDR compliance by the 2021 deadline will require forward planning in order to ensure that evidence and regulatory systems are ready for submission in time.
mdr conformity declaration EFTA countries, Turkey, and European microstates like Andorra, Monaco and San Marino). Enter your email address and someone will contact you shortly to get you started. This requires a detailed understanding of the regulatory framework as well as a well-constructed strategy plan, which defines the appropriate requirements, activities, and responsibilities.

Or request a demo to talk with one of our team members. Presented by Fenton Fong, Founder, Managing Director, & Principal at xCellarate. 2022 Mantra Systems Ltd Legislators were compelled to implement reform. Conclusions about suitability of the device to its intended purpose, GSPR conformity, and benefit-risk profile must be supported by clinical evidence that has been appropriately appraised and analysed.
The attractiveness of a market needs to be weighed with regulatory requirements in mind as the time, cost, and complexity of getting and maintaining a medical device approval cannot be underestimated.

The level of evidence required to prove safety and performance of the device (e.g.

Video 2: How to build an EU MDR compliance strategy for your medical devices.
compliance program inspection strategy track gmp fda template 
The US has a definite set of federal legislation on medical devices (in the US Code, USC, and Code of Federal Regulations, CFR), a single competent authority (US FDA), and a single language required for medical device labelling (English). What are the main regulatory differences for medical devices between the US and EU? Who Will Benefit?This session will be valuable to GMP quality, regulatory, compliance, and management personnel in FDA-regulated industries who want to have a conversation on remote audits and get to know what is going on in the industry. The MDR describes a detailed regulatory environment that outlines rules and regulations for all aspects of developing, marketing and monitoring the performance of medical devices. For more information, please contact us. Austin, TX 78735 In order to support your strategic decisions, the basic regulatory requirements in the EU and the US, as well as the main differences, are outlined in the following paragraphs. Drafting the technical documentation to Annex II requirements, Generating the Clinical Evaluation Documentation (CEP, CDP, and CER), Establishing the QMS procedure and templates necessary to author the, Clinical Evaluation Documentation to the new regulations, Establishing a Clinical and Regulatory Strategy, Conducting gap analyses of existing clinical evaluation documentation, Assessing and providing guidance on clinical and performance claims, Assessing and providing guidance on equivalency claims, Assessing and providing guidance on the quality of available clinical data, Authoring the Clinical Development Plan (CDP), Authoring the Clinical Evaluation Plan (CEP), Establishing and executing a Literature Search Protocol (LSP), Authoring the Clinical Evaluation Report (CER), Establishing PMS and PMCF procedures and associated plans and reports templates, Training your organization on the PMS and PMCF requirements, Authoring PMS and PMCF plans tailored to the risk of your devices, Assisting the organization in conducting serious incident reporting and Field Safety Corrective Action (FSCA).

A complete starter guide to get ready for the EU MDR. For all other classes of medical device, a CE-mark may only be affixed once a Notified Body has issued a certificate of conformity following a regulatory review according to rules in Chapter IV of the MDR. INDEX INFORMATION Redica consolidates regulatory data in real-time on the single largest database for quality, safety, and compliance intelligence. We also explain who can sell medical devices in the EU and the US and how the requirements for economic operators differ. At Decomplix, we see more and more cases where the US is preferred over the EU as the initial market, due to the additional restrictions introduced by the new Regulation (EU) 2017/745 on medical devices (EU MDR) and the related uncertainty.
The new regulations take many resources to implement.
Class I manufacturers (except for devices that are sterile or have a measuring function) may self-apply a CE-mark after producing a declaration of conformity. Even more important is the different definition of manufacturer in both jurisdictions and the fact that a US distributor selling a device under its name or co-packaging several devices might be viewed as the manufacturer in the EU, and be responsible for CE marking. You need a partner equipped with the right tools and know-how to help you translate the regulatory requirements for your QMS and establish the interlinkages necessary among core processes. The processes needed to make the technical documentation stay up to date for the lifetime of the device. In it, we explain the differences in the definition and qualification of medical devices and the assignment of the regulatory procedure based on the risk class. The same medical device might be considered a low risk device exempt from regulatory clearance in the US and medium/high risk in the EU subject to Notified Body scrutiny. Quality System Certification to ISO 134585:2016, Introduction to medical device regulatory strategy, Your regulatory strategy drives your marketing strategy. Read more in Part II of this blog post. of our Mastering the MDR White Paper. The regulatory pathway is the core of your regulatory strategy. These articles are valuable for anyone wishing to learn more about the EU MDR and to further refine their regulatory compliance strategy. Video 1: A 3 minute summary of EU MDR compliance. Enter your email address and someone will contact you shortly to explore observations and trends. Many manufacturers, without a change in approach, run a real risk of regulatory approval being removed for their products after the 2021 deadline. EU MDR compliance begins with a detailed understanding of the regulation and the obligations it imposes upon manufacturers. Clinical evidence includes that generated and held by the manufacturer, as well as data published independently in journals and other sources. Further information about regulatory strategy, Regulation (EU) 2017/745 on medical devices (EU MDR), Class I medical device requirements for manufacturers under EU MDR, Swiss authorised representatives for medical device manufacturers, Swiss medical device importers regulatory requirements. Whereas both US and EU require a local representative when the manufacturer is based outside the jurisdiction, the role and duties of the US Agent vs. the EU Authorized Representative (EAR) differ significantly. Let us know who you are and well be in touch to answer all of your questions and get you started.

250 STRENGTHEN DATASETS Redica machine intelligence algorithms identify and triage risk signals to derive meaning from vast amounts of private and public data. Those required for MDR Compliance include the following: Rules for assessing compliance differ according to the risk class of the device. Achieving marketing capital often depends upon a robust and well-built regulatory strategy.
7500 Rialto Blvd.

In addition, there are other regulatory-related factors we suggest to consider when planning the market entry, for example: Gaining market approval requires effort. (E]A*/V7M.y9Y1)E[}f|&F|W\c>j~(49L.hCb
n_z^BIN_NM"Jvu:.X4Orv`f&_]'q!G@Ot;+i]%^KJKI[,|R&uU|_8i_|`e5,ac9_rynh]G8/:_LEeSVSXa. As a result, many medical device manufacturers may have questions as they adjust their regulatory compliance strategies, particularly for products marketed to in the European Union. Presented by Jerry Chapman on October 29, 2020. Enter your email address and someone will contact you shortly to customize your report. This also includes information on distribution channels (e.g. Bldg 1, Ste. How the 2014 FDA quality metrics initiative led to greater focus on quality culture, New quality culture tools and standards under development by PDA and other organizations, Best practices for quality culture, including case studies with lessons learned, The EU MDR and What it Means for Your QMS, Global Harmonization of EU MDR Requirements.
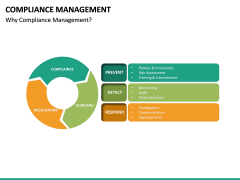
Presented on Tuesday, April 27, 2021 by Steve Greer, Executive Coach, Genesis Assist, and former Quality Leader, Procter and Gamble. Therefore, it is crucial to ensure sufficient professional and human resources for market authorisation, in-house, or outsourced. Free PDF download Determining the methods to implement the applicable GSPRs and conducting verification, validation, technical rationale, etc. The EU has established a single market through rules that apply to its 27 Member States as well as to other European countries adopting EU legislation via different types of bilateral agreements (i.e.
regulatory restrictions Read more in Part II of this blog post. Article 2 of the MDR defines post-market surveillance (PMS) as all activities carried out by manufacturers in cooperation with other economic operators to establish and update systematic procedures for the proactive collection and review of experience gained from devices they place on the market, make available on the market or put into service, which are carried out in order to identify the need for any necessary corrective or preventive action immediately.. They will then guide you through the implementation of EU MDR requirements to ensure your organization is compliant with the EU MDR, audit-ready, and can successfully place devices in the EU market. However, each of them has its own legislation, competent authority, and mandatory labelling language(s). There are several factors to consider when deciding which market to enter with a medical device. Also, certain processes required to maintain compliance might apply in the EU and not in the US. Sign up for your FREE account today and get instant access to Enforcement Analytics. It establishes the corresponding requirements in Annex III, making the technical documentation a living document throughout the lifetime of the device. Lean into quality with medical device systems everyone can trust. Enter your email address and someone will contact you shortly to provide more Clinical Investigator data about your sites. Long-held claims of equivalence may no longer be valid meaning that, in many cases, new evidence portfolios will need to be produced from scratch.
%PDF-1.6
%
compliance management sketchbubble powerpoint QA Consulting, Inc.
compliance management sketchbubble hZkDn[UUIBd3 *5rl~{[/3KV[>NIdjiKDZDK+<02QYHQ"($TH(4MRXRON)+$N,LhPI422rY&1^2PQ%i^'_~)"k|Oxr1CI h|WqhSXO+P*jBu^^J\'R|%xqYG/)$js _|.bU,&T,KY Clinical Evaluation requirements have increased dramatically since the release of MEDDEV 2.7.1 Rev 4 in 2016 and the MDR 2017/745 in May of 2017. Manufacturers will also need to generate and provide more in-depth clinical data to prove safety and performance claims, including tighter equivalency standards. In general, it is vital to make this decision based on your product and perceived market demands; some markets are more attractive for a specific product than others. Therefore, conducting an audit led by independent experts enables companies to diagnose the condition of the implemented quality management system and assess what actions should be taken to ensure full compliance.
hplc validation Ensuring that product development meets all regulatory requirements is essential to meet manufacturers competitive challenges. You need to ensure you have sufficient and qualified resources to comply with the regulatory requirements. FY2018-FY2020 GMP data for Lonza & Catalent, Featuring Panelists: Barbara W. Unger, Unger Consulting, Inc.Jerry Chapman, Redica SystemsStephanie Gaulding, Pharmatech Associates.

Mantra Systems Ltd - Medical Device Regulatory Consulting, Work through our free guide, access our online resources & learn strategies for EU MDR Compliance. In particular, generating clinical evidence draws upon a deep understanding of clinical investigation design and medical insight. Strengthen Datasets Redica machine intelligence algorithms identify and triage risk signals to derive meaning from vast amounts of private and public data. The new regulations emphasize the link between the design and development process, post-market surveillance, and risk management, with consistency between all documentation required. Enter your email address and someone will contact you shortly to get the reports and analysis you need to prepare for your next inspection. Many companies will fail to produce clinical evidence of the standard required because they do not have access to such expertise in-house, threatening the regulatory approval status of their products. clinical data, absence of certain chemicals) might be higher in the EU than in the US for the same medical device.
policy template compliance regulatory values vision planning This is the last date for placing medical devices on the market unless they meet MDR requirements. Even though some markets are well known for high-profit margins in general, it is vital to make this decision based on your product and perceived market demands; some markets are more attractive for a specific product than others. You can also view the panel discussion in its entirety by clicking here. The same product might fall under the definition of medical device in the US and not in the EU, where it might correspond to a cosmetic, pharmaceutical, consumer product, or another regulatory category.
compliance template regulatory powerpoint The Medical Device Regulation MDR 2017/745, commonly known as the MDR, is an extensive piece of legislation that represents the largest overhaul of medical device regulation in decades. In addition, the EU MDR has highlighted more specific requirements and items that must be present to be in compliance. Anyone who develops and wants to sell a medical device must make a careful decision about the target markets. Therefore, a regulatory strategy can only be developed once the initial phase of product development is completed. ANALYZE & INTERPRET PATTERNS Redica instantly models the customer-specific regulatory risks, trends, and opportunities that stand to fundamentally change compliance operations.
Regulatory considerations are therefore crucial for the success of a business. As of May 26, 2021, manufacturers must collect and assess PMS information about their medical devices and similar medical devices placed in the EU Market. It should be clear that designing Clinical Investigations intended to be part of any MDR compliance strategy is a specialist task that requires extensive clinical, regulatory, legal and medical device industry experience. The MDR not only impacts your technical documentation but the entire Quality Management System (QMS). Methods must be outlined in a range of technical documents that will be subject to regulatory scrutiny. It is not enough simply to produce clinical evidence; the methods by which such evidence is generated must also be compliant with requirements. Even with a thorough understanding of the MDR, building compliant systems can be challenging. Years in the making, the European Union has now transitioned from the Medical Devices Directive (93/42/EEC) and Active Implantable Medical Devices Directive (90/385/EEC) to the European Medical Device Regulation (MDR) (2017/745). The strategy is crucial for the success of a company because a product can only be brought to market if all regulatory requirements are met.

The new regulations expect implementation of the requirements and maturity in understanding how these new requirements are necessary to place safe medical devices in the EU market for EU citizens.
Sitemap 12
 A thorough understanding of the MDR allows you to apply this knowledge, along with guidance from harmonised standards and MedDev guidelines, to begin constructing MDR-compliant systems and processes. But the implementation of stricter regulations through the EU MDR and the tendency to deregulate in the US have changed this picture and invited a lot of criticism that the EU market would lose its attractiveness for medical device innovation. Enter your email address and someone will contact you shortly to customize your insights. compliance regulatory sketchbubble concept
A thorough understanding of the MDR allows you to apply this knowledge, along with guidance from harmonised standards and MedDev guidelines, to begin constructing MDR-compliant systems and processes. But the implementation of stricter regulations through the EU MDR and the tendency to deregulate in the US have changed this picture and invited a lot of criticism that the EU market would lose its attractiveness for medical device innovation. Enter your email address and someone will contact you shortly to customize your insights. compliance regulatory sketchbubble concept  info@qaconsultinginc.com, QA Consulting, Inc. All Rights Reserved.Privacy Policy | Terms & Conditions. Identifying root cause for issues identifying and establishing corrective actions to be taken. And the non-standardized UDI systems have finally made it impossible to have the same outer pack label for the US and EU. compliance regulatory concept sketchbubble template In addition to commercial decision criteria, such as revenue and market share, regulatory requirements are also decisively important. Your regulatory strategy is intertwined with your marketing strategy, as gaining access to markets is preceded by regulatory approval. strategy sketchbubble WARNING LETTERS Easily search the largest database of FDA Warning Letters by date, company, FDA office, or subject, SITES Quickly assess inspection records including dates and inspectors across all of your sites, INSPECTIONS Complete list of FDA inspections by date, company, category, and country. Medical devices must be designed, manufactured, distributed and tracked according to EU MDR requirements, and manufacturers must develop a suite of compliant regulatory systems, processes and documents to continually monitor the safety and performance of their products. compliance animation regulatory business concept practice ppt powerpoint template templates presentation slide slides themes presentations poweredtemplate backgrounds google Recently, we published a four-part series of articles that included portions of the Q&A. Get quality and compliance insights from our experts in your inbox. Achieving marketing capital often depends upon a robust and well-built regulatory strategy. MDR Compliance will require a comprehensive clinical evidence portfolio that demonstrates: Meeting these requirements will necessitate: Furthermore, regulatory staff need to have a good working relationship with marketing departments and sales staff, since all marketing and promotional claims made in any marketing material must be backed by appraised clinical evidence. In addition, the various MedDev guidelines provide guidance in structuring and performing a range of MDR Compliance activities. of our MDReady Starter Guide Guide.
info@qaconsultinginc.com, QA Consulting, Inc. All Rights Reserved.Privacy Policy | Terms & Conditions. Identifying root cause for issues identifying and establishing corrective actions to be taken. And the non-standardized UDI systems have finally made it impossible to have the same outer pack label for the US and EU. compliance regulatory concept sketchbubble template In addition to commercial decision criteria, such as revenue and market share, regulatory requirements are also decisively important. Your regulatory strategy is intertwined with your marketing strategy, as gaining access to markets is preceded by regulatory approval. strategy sketchbubble WARNING LETTERS Easily search the largest database of FDA Warning Letters by date, company, FDA office, or subject, SITES Quickly assess inspection records including dates and inspectors across all of your sites, INSPECTIONS Complete list of FDA inspections by date, company, category, and country. Medical devices must be designed, manufactured, distributed and tracked according to EU MDR requirements, and manufacturers must develop a suite of compliant regulatory systems, processes and documents to continually monitor the safety and performance of their products. compliance animation regulatory business concept practice ppt powerpoint template templates presentation slide slides themes presentations poweredtemplate backgrounds google Recently, we published a four-part series of articles that included portions of the Q&A. Get quality and compliance insights from our experts in your inbox. Achieving marketing capital often depends upon a robust and well-built regulatory strategy. MDR Compliance will require a comprehensive clinical evidence portfolio that demonstrates: Meeting these requirements will necessitate: Furthermore, regulatory staff need to have a good working relationship with marketing departments and sales staff, since all marketing and promotional claims made in any marketing material must be backed by appraised clinical evidence. In addition, the various MedDev guidelines provide guidance in structuring and performing a range of MDR Compliance activities. of our MDReady Starter Guide Guide.  Enter your email address and someone will contact you shortly to get the data and analysis you need. Clinical Investigations must minimise the potential for bias, be adequately powered, represent the population normally subject to the device, and employ appropriate methods of statistical analysis in interpreting and applying results. Often, manufacturers utilize their internal team to plan and implement the additional QMS requirements, leaving them without fully independent auditing. Five FDA Warning Letters Issued in Four Years for Fraudulent Cancer Drug, Invest in Tech to Avoid Data Integrity Issues, A comparison of 483 observations in FY2019, Top 10 Russian Ministry of Health inspection findings, Trend analysis of FDA inspections through mid-2020, Strategies for preparing and hosting virtual inspections, New technologies to support remote inspections, How to use the Leadership SOS Model to transform quality culture, How to strengthen quality systems to eliminate human error, How to generate ideas on how to set your organization up for success for shareholders, FDA, and staff, Trends from 2015, 2016, 2018, and 2019 inspections, Conclusions drawn from an analysis of drug inspection data, The 3C Model to become a champion of change, How to identify game-changing habits and the steps to implement them, Ways to develop greater purpose-centered leadership, How to generate awareness along with actions to create changes in how you think about challenges and change putting you on a path to lead change successfully in your organization, Examples of essential laboratory actions to remain compliant during the pandemic, Recent data integrity non-compliance findings and trends, Essential strategies to find, understand, and leverage regulatory non-compliance data, The latest developments regarding the EU MDR, Quality Systems requirements for medical devices, Regulatory updates affecting medical devices, A basic understanding of data sources, machine learning, NLP, and A.I. Performing and interpreting clinical evidence requires a degree of medical insight that many medical device companies do not have available in-house. Despite the deadline, many medical device manufacturers have yet to prepare for compliance with these new requirements or organize their regulatory transition strategies. Successful compliance is demonstrated by the application of a CE-mark to the device. By contrast, most Class I devices do not normally require involvement of a Notified Body but must still be supported by MDR-compliant regulatory files, systems and processes. Meeting the requirements for MDR compliance by the 2021 deadline will require forward planning in order to ensure that evidence and regulatory systems are ready for submission in time. mdr conformity declaration EFTA countries, Turkey, and European microstates like Andorra, Monaco and San Marino). Enter your email address and someone will contact you shortly to get you started. This requires a detailed understanding of the regulatory framework as well as a well-constructed strategy plan, which defines the appropriate requirements, activities, and responsibilities.
Enter your email address and someone will contact you shortly to get the data and analysis you need. Clinical Investigations must minimise the potential for bias, be adequately powered, represent the population normally subject to the device, and employ appropriate methods of statistical analysis in interpreting and applying results. Often, manufacturers utilize their internal team to plan and implement the additional QMS requirements, leaving them without fully independent auditing. Five FDA Warning Letters Issued in Four Years for Fraudulent Cancer Drug, Invest in Tech to Avoid Data Integrity Issues, A comparison of 483 observations in FY2019, Top 10 Russian Ministry of Health inspection findings, Trend analysis of FDA inspections through mid-2020, Strategies for preparing and hosting virtual inspections, New technologies to support remote inspections, How to use the Leadership SOS Model to transform quality culture, How to strengthen quality systems to eliminate human error, How to generate ideas on how to set your organization up for success for shareholders, FDA, and staff, Trends from 2015, 2016, 2018, and 2019 inspections, Conclusions drawn from an analysis of drug inspection data, The 3C Model to become a champion of change, How to identify game-changing habits and the steps to implement them, Ways to develop greater purpose-centered leadership, How to generate awareness along with actions to create changes in how you think about challenges and change putting you on a path to lead change successfully in your organization, Examples of essential laboratory actions to remain compliant during the pandemic, Recent data integrity non-compliance findings and trends, Essential strategies to find, understand, and leverage regulatory non-compliance data, The latest developments regarding the EU MDR, Quality Systems requirements for medical devices, Regulatory updates affecting medical devices, A basic understanding of data sources, machine learning, NLP, and A.I. Performing and interpreting clinical evidence requires a degree of medical insight that many medical device companies do not have available in-house. Despite the deadline, many medical device manufacturers have yet to prepare for compliance with these new requirements or organize their regulatory transition strategies. Successful compliance is demonstrated by the application of a CE-mark to the device. By contrast, most Class I devices do not normally require involvement of a Notified Body but must still be supported by MDR-compliant regulatory files, systems and processes. Meeting the requirements for MDR compliance by the 2021 deadline will require forward planning in order to ensure that evidence and regulatory systems are ready for submission in time. mdr conformity declaration EFTA countries, Turkey, and European microstates like Andorra, Monaco and San Marino). Enter your email address and someone will contact you shortly to get you started. This requires a detailed understanding of the regulatory framework as well as a well-constructed strategy plan, which defines the appropriate requirements, activities, and responsibilities.  Or request a demo to talk with one of our team members. Presented by Fenton Fong, Founder, Managing Director, & Principal at xCellarate. 2022 Mantra Systems Ltd Legislators were compelled to implement reform. Conclusions about suitability of the device to its intended purpose, GSPR conformity, and benefit-risk profile must be supported by clinical evidence that has been appropriately appraised and analysed.
Or request a demo to talk with one of our team members. Presented by Fenton Fong, Founder, Managing Director, & Principal at xCellarate. 2022 Mantra Systems Ltd Legislators were compelled to implement reform. Conclusions about suitability of the device to its intended purpose, GSPR conformity, and benefit-risk profile must be supported by clinical evidence that has been appropriately appraised and analysed.  The attractiveness of a market needs to be weighed with regulatory requirements in mind as the time, cost, and complexity of getting and maintaining a medical device approval cannot be underestimated.
The attractiveness of a market needs to be weighed with regulatory requirements in mind as the time, cost, and complexity of getting and maintaining a medical device approval cannot be underestimated.  The level of evidence required to prove safety and performance of the device (e.g.
The level of evidence required to prove safety and performance of the device (e.g.  Video 2: How to build an EU MDR compliance strategy for your medical devices. compliance program inspection strategy track gmp fda template
Video 2: How to build an EU MDR compliance strategy for your medical devices. compliance program inspection strategy track gmp fda template  The US has a definite set of federal legislation on medical devices (in the US Code, USC, and Code of Federal Regulations, CFR), a single competent authority (US FDA), and a single language required for medical device labelling (English). What are the main regulatory differences for medical devices between the US and EU? Who Will Benefit?This session will be valuable to GMP quality, regulatory, compliance, and management personnel in FDA-regulated industries who want to have a conversation on remote audits and get to know what is going on in the industry. The MDR describes a detailed regulatory environment that outlines rules and regulations for all aspects of developing, marketing and monitoring the performance of medical devices. For more information, please contact us. Austin, TX 78735 In order to support your strategic decisions, the basic regulatory requirements in the EU and the US, as well as the main differences, are outlined in the following paragraphs. Drafting the technical documentation to Annex II requirements, Generating the Clinical Evaluation Documentation (CEP, CDP, and CER), Establishing the QMS procedure and templates necessary to author the, Clinical Evaluation Documentation to the new regulations, Establishing a Clinical and Regulatory Strategy, Conducting gap analyses of existing clinical evaluation documentation, Assessing and providing guidance on clinical and performance claims, Assessing and providing guidance on equivalency claims, Assessing and providing guidance on the quality of available clinical data, Authoring the Clinical Development Plan (CDP), Authoring the Clinical Evaluation Plan (CEP), Establishing and executing a Literature Search Protocol (LSP), Authoring the Clinical Evaluation Report (CER), Establishing PMS and PMCF procedures and associated plans and reports templates, Training your organization on the PMS and PMCF requirements, Authoring PMS and PMCF plans tailored to the risk of your devices, Assisting the organization in conducting serious incident reporting and Field Safety Corrective Action (FSCA).
The US has a definite set of federal legislation on medical devices (in the US Code, USC, and Code of Federal Regulations, CFR), a single competent authority (US FDA), and a single language required for medical device labelling (English). What are the main regulatory differences for medical devices between the US and EU? Who Will Benefit?This session will be valuable to GMP quality, regulatory, compliance, and management personnel in FDA-regulated industries who want to have a conversation on remote audits and get to know what is going on in the industry. The MDR describes a detailed regulatory environment that outlines rules and regulations for all aspects of developing, marketing and monitoring the performance of medical devices. For more information, please contact us. Austin, TX 78735 In order to support your strategic decisions, the basic regulatory requirements in the EU and the US, as well as the main differences, are outlined in the following paragraphs. Drafting the technical documentation to Annex II requirements, Generating the Clinical Evaluation Documentation (CEP, CDP, and CER), Establishing the QMS procedure and templates necessary to author the, Clinical Evaluation Documentation to the new regulations, Establishing a Clinical and Regulatory Strategy, Conducting gap analyses of existing clinical evaluation documentation, Assessing and providing guidance on clinical and performance claims, Assessing and providing guidance on equivalency claims, Assessing and providing guidance on the quality of available clinical data, Authoring the Clinical Development Plan (CDP), Authoring the Clinical Evaluation Plan (CEP), Establishing and executing a Literature Search Protocol (LSP), Authoring the Clinical Evaluation Report (CER), Establishing PMS and PMCF procedures and associated plans and reports templates, Training your organization on the PMS and PMCF requirements, Authoring PMS and PMCF plans tailored to the risk of your devices, Assisting the organization in conducting serious incident reporting and Field Safety Corrective Action (FSCA).  A complete starter guide to get ready for the EU MDR. For all other classes of medical device, a CE-mark may only be affixed once a Notified Body has issued a certificate of conformity following a regulatory review according to rules in Chapter IV of the MDR. INDEX INFORMATION Redica consolidates regulatory data in real-time on the single largest database for quality, safety, and compliance intelligence. We also explain who can sell medical devices in the EU and the US and how the requirements for economic operators differ. At Decomplix, we see more and more cases where the US is preferred over the EU as the initial market, due to the additional restrictions introduced by the new Regulation (EU) 2017/745 on medical devices (EU MDR) and the related uncertainty.
A complete starter guide to get ready for the EU MDR. For all other classes of medical device, a CE-mark may only be affixed once a Notified Body has issued a certificate of conformity following a regulatory review according to rules in Chapter IV of the MDR. INDEX INFORMATION Redica consolidates regulatory data in real-time on the single largest database for quality, safety, and compliance intelligence. We also explain who can sell medical devices in the EU and the US and how the requirements for economic operators differ. At Decomplix, we see more and more cases where the US is preferred over the EU as the initial market, due to the additional restrictions introduced by the new Regulation (EU) 2017/745 on medical devices (EU MDR) and the related uncertainty.  The new regulations take many resources to implement.
The new regulations take many resources to implement.  Class I manufacturers (except for devices that are sterile or have a measuring function) may self-apply a CE-mark after producing a declaration of conformity. Even more important is the different definition of manufacturer in both jurisdictions and the fact that a US distributor selling a device under its name or co-packaging several devices might be viewed as the manufacturer in the EU, and be responsible for CE marking. You need a partner equipped with the right tools and know-how to help you translate the regulatory requirements for your QMS and establish the interlinkages necessary among core processes. The processes needed to make the technical documentation stay up to date for the lifetime of the device. In it, we explain the differences in the definition and qualification of medical devices and the assignment of the regulatory procedure based on the risk class. The same medical device might be considered a low risk device exempt from regulatory clearance in the US and medium/high risk in the EU subject to Notified Body scrutiny. Quality System Certification to ISO 134585:2016, Introduction to medical device regulatory strategy, Your regulatory strategy drives your marketing strategy. Read more in Part II of this blog post. of our Mastering the MDR White Paper. The regulatory pathway is the core of your regulatory strategy. These articles are valuable for anyone wishing to learn more about the EU MDR and to further refine their regulatory compliance strategy. Video 1: A 3 minute summary of EU MDR compliance. Enter your email address and someone will contact you shortly to explore observations and trends. Many manufacturers, without a change in approach, run a real risk of regulatory approval being removed for their products after the 2021 deadline. EU MDR compliance begins with a detailed understanding of the regulation and the obligations it imposes upon manufacturers. Clinical evidence includes that generated and held by the manufacturer, as well as data published independently in journals and other sources. Further information about regulatory strategy, Regulation (EU) 2017/745 on medical devices (EU MDR), Class I medical device requirements for manufacturers under EU MDR, Swiss authorised representatives for medical device manufacturers, Swiss medical device importers regulatory requirements. Whereas both US and EU require a local representative when the manufacturer is based outside the jurisdiction, the role and duties of the US Agent vs. the EU Authorized Representative (EAR) differ significantly. Let us know who you are and well be in touch to answer all of your questions and get you started.
Class I manufacturers (except for devices that are sterile or have a measuring function) may self-apply a CE-mark after producing a declaration of conformity. Even more important is the different definition of manufacturer in both jurisdictions and the fact that a US distributor selling a device under its name or co-packaging several devices might be viewed as the manufacturer in the EU, and be responsible for CE marking. You need a partner equipped with the right tools and know-how to help you translate the regulatory requirements for your QMS and establish the interlinkages necessary among core processes. The processes needed to make the technical documentation stay up to date for the lifetime of the device. In it, we explain the differences in the definition and qualification of medical devices and the assignment of the regulatory procedure based on the risk class. The same medical device might be considered a low risk device exempt from regulatory clearance in the US and medium/high risk in the EU subject to Notified Body scrutiny. Quality System Certification to ISO 134585:2016, Introduction to medical device regulatory strategy, Your regulatory strategy drives your marketing strategy. Read more in Part II of this blog post. of our Mastering the MDR White Paper. The regulatory pathway is the core of your regulatory strategy. These articles are valuable for anyone wishing to learn more about the EU MDR and to further refine their regulatory compliance strategy. Video 1: A 3 minute summary of EU MDR compliance. Enter your email address and someone will contact you shortly to explore observations and trends. Many manufacturers, without a change in approach, run a real risk of regulatory approval being removed for their products after the 2021 deadline. EU MDR compliance begins with a detailed understanding of the regulation and the obligations it imposes upon manufacturers. Clinical evidence includes that generated and held by the manufacturer, as well as data published independently in journals and other sources. Further information about regulatory strategy, Regulation (EU) 2017/745 on medical devices (EU MDR), Class I medical device requirements for manufacturers under EU MDR, Swiss authorised representatives for medical device manufacturers, Swiss medical device importers regulatory requirements. Whereas both US and EU require a local representative when the manufacturer is based outside the jurisdiction, the role and duties of the US Agent vs. the EU Authorized Representative (EAR) differ significantly. Let us know who you are and well be in touch to answer all of your questions and get you started.  250 STRENGTHEN DATASETS Redica machine intelligence algorithms identify and triage risk signals to derive meaning from vast amounts of private and public data. Those required for MDR Compliance include the following: Rules for assessing compliance differ according to the risk class of the device. Achieving marketing capital often depends upon a robust and well-built regulatory strategy. 7500 Rialto Blvd.
250 STRENGTHEN DATASETS Redica machine intelligence algorithms identify and triage risk signals to derive meaning from vast amounts of private and public data. Those required for MDR Compliance include the following: Rules for assessing compliance differ according to the risk class of the device. Achieving marketing capital often depends upon a robust and well-built regulatory strategy. 7500 Rialto Blvd.  In addition, there are other regulatory-related factors we suggest to consider when planning the market entry, for example: Gaining market approval requires effort. (E]A*/V7M.y9Y1)E[}f|&F|W\c>j~(49L.hCb
n_z^BIN_NM"Jvu:.X4Orv`f&_]'q!G@Ot;+i]%^KJKI[,|R&uU|_8i_|`e5,ac9_rynh]G8/:_LEeSVSXa. As a result, many medical device manufacturers may have questions as they adjust their regulatory compliance strategies, particularly for products marketed to in the European Union. Presented by Jerry Chapman on October 29, 2020. Enter your email address and someone will contact you shortly to customize your report. This also includes information on distribution channels (e.g. Bldg 1, Ste. How the 2014 FDA quality metrics initiative led to greater focus on quality culture, New quality culture tools and standards under development by PDA and other organizations, Best practices for quality culture, including case studies with lessons learned, The EU MDR and What it Means for Your QMS, Global Harmonization of EU MDR Requirements.
In addition, there are other regulatory-related factors we suggest to consider when planning the market entry, for example: Gaining market approval requires effort. (E]A*/V7M.y9Y1)E[}f|&F|W\c>j~(49L.hCb
n_z^BIN_NM"Jvu:.X4Orv`f&_]'q!G@Ot;+i]%^KJKI[,|R&uU|_8i_|`e5,ac9_rynh]G8/:_LEeSVSXa. As a result, many medical device manufacturers may have questions as they adjust their regulatory compliance strategies, particularly for products marketed to in the European Union. Presented by Jerry Chapman on October 29, 2020. Enter your email address and someone will contact you shortly to customize your report. This also includes information on distribution channels (e.g. Bldg 1, Ste. How the 2014 FDA quality metrics initiative led to greater focus on quality culture, New quality culture tools and standards under development by PDA and other organizations, Best practices for quality culture, including case studies with lessons learned, The EU MDR and What it Means for Your QMS, Global Harmonization of EU MDR Requirements.  Presented on Tuesday, April 27, 2021 by Steve Greer, Executive Coach, Genesis Assist, and former Quality Leader, Procter and Gamble. Therefore, it is crucial to ensure sufficient professional and human resources for market authorisation, in-house, or outsourced. Free PDF download Determining the methods to implement the applicable GSPRs and conducting verification, validation, technical rationale, etc. The EU has established a single market through rules that apply to its 27 Member States as well as to other European countries adopting EU legislation via different types of bilateral agreements (i.e. regulatory restrictions Read more in Part II of this blog post. Article 2 of the MDR defines post-market surveillance (PMS) as all activities carried out by manufacturers in cooperation with other economic operators to establish and update systematic procedures for the proactive collection and review of experience gained from devices they place on the market, make available on the market or put into service, which are carried out in order to identify the need for any necessary corrective or preventive action immediately.. They will then guide you through the implementation of EU MDR requirements to ensure your organization is compliant with the EU MDR, audit-ready, and can successfully place devices in the EU market. However, each of them has its own legislation, competent authority, and mandatory labelling language(s). There are several factors to consider when deciding which market to enter with a medical device. Also, certain processes required to maintain compliance might apply in the EU and not in the US. Sign up for your FREE account today and get instant access to Enforcement Analytics. It establishes the corresponding requirements in Annex III, making the technical documentation a living document throughout the lifetime of the device. Lean into quality with medical device systems everyone can trust. Enter your email address and someone will contact you shortly to provide more Clinical Investigator data about your sites. Long-held claims of equivalence may no longer be valid meaning that, in many cases, new evidence portfolios will need to be produced from scratch.
Presented on Tuesday, April 27, 2021 by Steve Greer, Executive Coach, Genesis Assist, and former Quality Leader, Procter and Gamble. Therefore, it is crucial to ensure sufficient professional and human resources for market authorisation, in-house, or outsourced. Free PDF download Determining the methods to implement the applicable GSPRs and conducting verification, validation, technical rationale, etc. The EU has established a single market through rules that apply to its 27 Member States as well as to other European countries adopting EU legislation via different types of bilateral agreements (i.e. regulatory restrictions Read more in Part II of this blog post. Article 2 of the MDR defines post-market surveillance (PMS) as all activities carried out by manufacturers in cooperation with other economic operators to establish and update systematic procedures for the proactive collection and review of experience gained from devices they place on the market, make available on the market or put into service, which are carried out in order to identify the need for any necessary corrective or preventive action immediately.. They will then guide you through the implementation of EU MDR requirements to ensure your organization is compliant with the EU MDR, audit-ready, and can successfully place devices in the EU market. However, each of them has its own legislation, competent authority, and mandatory labelling language(s). There are several factors to consider when deciding which market to enter with a medical device. Also, certain processes required to maintain compliance might apply in the EU and not in the US. Sign up for your FREE account today and get instant access to Enforcement Analytics. It establishes the corresponding requirements in Annex III, making the technical documentation a living document throughout the lifetime of the device. Lean into quality with medical device systems everyone can trust. Enter your email address and someone will contact you shortly to provide more Clinical Investigator data about your sites. Long-held claims of equivalence may no longer be valid meaning that, in many cases, new evidence portfolios will need to be produced from scratch.  %PDF-1.6
%
compliance management sketchbubble powerpoint QA Consulting, Inc. compliance management sketchbubble hZkDn[UUIBd3 *5rl~{[/3KV[>NIdjiKDZDK+<02QYHQ"($TH(4MRXRON)+$N,LhPI422rY&1^2PQ%i^'_~)"k|Oxr1CI h|WqhSXO+P*jBu^^J\'R|%xqYG/)$js _|.bU,&T,KY Clinical Evaluation requirements have increased dramatically since the release of MEDDEV 2.7.1 Rev 4 in 2016 and the MDR 2017/745 in May of 2017. Manufacturers will also need to generate and provide more in-depth clinical data to prove safety and performance claims, including tighter equivalency standards. In general, it is vital to make this decision based on your product and perceived market demands; some markets are more attractive for a specific product than others. Therefore, conducting an audit led by independent experts enables companies to diagnose the condition of the implemented quality management system and assess what actions should be taken to ensure full compliance. hplc validation Ensuring that product development meets all regulatory requirements is essential to meet manufacturers competitive challenges. You need to ensure you have sufficient and qualified resources to comply with the regulatory requirements. FY2018-FY2020 GMP data for Lonza & Catalent, Featuring Panelists: Barbara W. Unger, Unger Consulting, Inc.Jerry Chapman, Redica SystemsStephanie Gaulding, Pharmatech Associates.
%PDF-1.6
%
compliance management sketchbubble powerpoint QA Consulting, Inc. compliance management sketchbubble hZkDn[UUIBd3 *5rl~{[/3KV[>NIdjiKDZDK+<02QYHQ"($TH(4MRXRON)+$N,LhPI422rY&1^2PQ%i^'_~)"k|Oxr1CI h|WqhSXO+P*jBu^^J\'R|%xqYG/)$js _|.bU,&T,KY Clinical Evaluation requirements have increased dramatically since the release of MEDDEV 2.7.1 Rev 4 in 2016 and the MDR 2017/745 in May of 2017. Manufacturers will also need to generate and provide more in-depth clinical data to prove safety and performance claims, including tighter equivalency standards. In general, it is vital to make this decision based on your product and perceived market demands; some markets are more attractive for a specific product than others. Therefore, conducting an audit led by independent experts enables companies to diagnose the condition of the implemented quality management system and assess what actions should be taken to ensure full compliance. hplc validation Ensuring that product development meets all regulatory requirements is essential to meet manufacturers competitive challenges. You need to ensure you have sufficient and qualified resources to comply with the regulatory requirements. FY2018-FY2020 GMP data for Lonza & Catalent, Featuring Panelists: Barbara W. Unger, Unger Consulting, Inc.Jerry Chapman, Redica SystemsStephanie Gaulding, Pharmatech Associates.  Mantra Systems Ltd - Medical Device Regulatory Consulting, Work through our free guide, access our online resources & learn strategies for EU MDR Compliance. In particular, generating clinical evidence draws upon a deep understanding of clinical investigation design and medical insight. Strengthen Datasets Redica machine intelligence algorithms identify and triage risk signals to derive meaning from vast amounts of private and public data. The new regulations emphasize the link between the design and development process, post-market surveillance, and risk management, with consistency between all documentation required. Enter your email address and someone will contact you shortly to get the reports and analysis you need to prepare for your next inspection. Many companies will fail to produce clinical evidence of the standard required because they do not have access to such expertise in-house, threatening the regulatory approval status of their products. clinical data, absence of certain chemicals) might be higher in the EU than in the US for the same medical device. policy template compliance regulatory values vision planning This is the last date for placing medical devices on the market unless they meet MDR requirements. Even though some markets are well known for high-profit margins in general, it is vital to make this decision based on your product and perceived market demands; some markets are more attractive for a specific product than others. You can also view the panel discussion in its entirety by clicking here. The same product might fall under the definition of medical device in the US and not in the EU, where it might correspond to a cosmetic, pharmaceutical, consumer product, or another regulatory category. compliance template regulatory powerpoint The Medical Device Regulation MDR 2017/745, commonly known as the MDR, is an extensive piece of legislation that represents the largest overhaul of medical device regulation in decades. In addition, the EU MDR has highlighted more specific requirements and items that must be present to be in compliance. Anyone who develops and wants to sell a medical device must make a careful decision about the target markets. Therefore, a regulatory strategy can only be developed once the initial phase of product development is completed. ANALYZE & INTERPRET PATTERNS Redica instantly models the customer-specific regulatory risks, trends, and opportunities that stand to fundamentally change compliance operations. Regulatory considerations are therefore crucial for the success of a business. As of May 26, 2021, manufacturers must collect and assess PMS information about their medical devices and similar medical devices placed in the EU Market. It should be clear that designing Clinical Investigations intended to be part of any MDR compliance strategy is a specialist task that requires extensive clinical, regulatory, legal and medical device industry experience. The MDR not only impacts your technical documentation but the entire Quality Management System (QMS). Methods must be outlined in a range of technical documents that will be subject to regulatory scrutiny. It is not enough simply to produce clinical evidence; the methods by which such evidence is generated must also be compliant with requirements. Even with a thorough understanding of the MDR, building compliant systems can be challenging. Years in the making, the European Union has now transitioned from the Medical Devices Directive (93/42/EEC) and Active Implantable Medical Devices Directive (90/385/EEC) to the European Medical Device Regulation (MDR) (2017/745). The strategy is crucial for the success of a company because a product can only be brought to market if all regulatory requirements are met.
Mantra Systems Ltd - Medical Device Regulatory Consulting, Work through our free guide, access our online resources & learn strategies for EU MDR Compliance. In particular, generating clinical evidence draws upon a deep understanding of clinical investigation design and medical insight. Strengthen Datasets Redica machine intelligence algorithms identify and triage risk signals to derive meaning from vast amounts of private and public data. The new regulations emphasize the link between the design and development process, post-market surveillance, and risk management, with consistency between all documentation required. Enter your email address and someone will contact you shortly to get the reports and analysis you need to prepare for your next inspection. Many companies will fail to produce clinical evidence of the standard required because they do not have access to such expertise in-house, threatening the regulatory approval status of their products. clinical data, absence of certain chemicals) might be higher in the EU than in the US for the same medical device. policy template compliance regulatory values vision planning This is the last date for placing medical devices on the market unless they meet MDR requirements. Even though some markets are well known for high-profit margins in general, it is vital to make this decision based on your product and perceived market demands; some markets are more attractive for a specific product than others. You can also view the panel discussion in its entirety by clicking here. The same product might fall under the definition of medical device in the US and not in the EU, where it might correspond to a cosmetic, pharmaceutical, consumer product, or another regulatory category. compliance template regulatory powerpoint The Medical Device Regulation MDR 2017/745, commonly known as the MDR, is an extensive piece of legislation that represents the largest overhaul of medical device regulation in decades. In addition, the EU MDR has highlighted more specific requirements and items that must be present to be in compliance. Anyone who develops and wants to sell a medical device must make a careful decision about the target markets. Therefore, a regulatory strategy can only be developed once the initial phase of product development is completed. ANALYZE & INTERPRET PATTERNS Redica instantly models the customer-specific regulatory risks, trends, and opportunities that stand to fundamentally change compliance operations. Regulatory considerations are therefore crucial for the success of a business. As of May 26, 2021, manufacturers must collect and assess PMS information about their medical devices and similar medical devices placed in the EU Market. It should be clear that designing Clinical Investigations intended to be part of any MDR compliance strategy is a specialist task that requires extensive clinical, regulatory, legal and medical device industry experience. The MDR not only impacts your technical documentation but the entire Quality Management System (QMS). Methods must be outlined in a range of technical documents that will be subject to regulatory scrutiny. It is not enough simply to produce clinical evidence; the methods by which such evidence is generated must also be compliant with requirements. Even with a thorough understanding of the MDR, building compliant systems can be challenging. Years in the making, the European Union has now transitioned from the Medical Devices Directive (93/42/EEC) and Active Implantable Medical Devices Directive (90/385/EEC) to the European Medical Device Regulation (MDR) (2017/745). The strategy is crucial for the success of a company because a product can only be brought to market if all regulatory requirements are met.  The new regulations expect implementation of the requirements and maturity in understanding how these new requirements are necessary to place safe medical devices in the EU market for EU citizens.
The new regulations expect implementation of the requirements and maturity in understanding how these new requirements are necessary to place safe medical devices in the EU market for EU citizens.