Overdose of salicylates (Aspirin) can cause MAC as well. Finally, low bicarbonate blood levels can result from elevated levels of ketones (common in unmanaged diabetes mellitus), which bind bicarbonate in the filtrate and prevent its conservation. The respiratory tract can adjust the blood pH upward in minutes by exhaling CO2 from the body. Aldosterone promotes (1) excretion of H+ and K+ in the distal tubule and the collecting duct and (2) reabsorption of the sodium (and water). Anion gap is a quantity which is almost equal to the sum of concentrations of unmeasurable anions (albumin plasma proteins, phosphates, sulphates, organic anions). 0000043851 00000 n
Thus you should notice that even alkalic pH (e.g. Value of pH in arterial blood higher than 7,8, resp. 4) Renal insufficiency leads to condition when normally excreted acids are cumulated (sulphates, phosphates, some other anions). Acidosis leads to efflux of K+ from the cells.
lysis extraction reagent purification neuromuscular disorders, CNS disorders, intoxications (opiates), asthmatic paroxysm), 4) Thorax movement restriction (e.g. A person who is diabetic and uses insulin can initiate ketoacidosis if a dose of insulin is missed. 0000145944 00000 n
Now we mention some particular states that lead to MAL: 1) Vomiting loss of HCl (thus loss of H+). Bicarbonate reabsorption takes place in proximal tubule cells. Since carbonic acid is very unstable molecule measurement of its concentration is very difficult. breathing in lack of oxygen). in the urine there is thousand times higher concentration of protons than in the cells/blood.


Respiratory alkalosis is caused by hyperventilation. This is provided by larger excretion of HCO3 by the kidneys.
phosphate buffer plasma system proteins intracellular compartments important both which basicmedicalkey Acid-balance balance is measured using the pH scale, as shown in Figure 26.4.1. This method is unfortunately dependent on accuracy of the measurements. Carbon dioxide is normally eliminated from the body by the respiratory system. Carbonic acid dissociates to H+ and HCO3. Ions H+ needed for this reaction are provided from non-bicarbonate buffers. Then we measure: 2) 3-hydroxybutyrate in diabetic ketoacidosis, 3) Phosphates and sulphates in renal failure.

They are continually produced by metabolism (incomplete oxidation of TAG, carbohydrates, proteins). Hypoproteinemia is caused by liver failure, nephrotic syndrome or malnutrition. Human body is evolutionary capable to handle acid load. Alkalosis is au contraire process that leads to the increase in pH value. 0000043483 00000 n
# $ F0] 9& JFIF H H Exif MM * b j( 1 r2 i H H Adobe Photoshop 7.0 2006:01:31 11:57:13 m ( &
H H JFIF H H Adobe_CM Adobe d Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and carbonic acid.
pbrp tfiib iib mcb 
0000144708 00000 n
electrophoresis sds discontinuous proteins protein edu disc socratic umbc userpages 
Therefore when there is increased AG it indicates that commonly non-measured acids accumulated. Thus greater AG indicates acidosis. 0000142387 00000 n
Bicarbonates are transported to the blood, whilst ammonium ions are excreted to the blood. That leads to hypokalemia. Base excess is defined as number of moles of strong acid that is needed to add to one litre of fully oxygenated blood to achieve pH 7,4 when pCO2 is 5,3 kPa and temperature is 37C. Rebreathing exhaled air will rapidly bring blood pH down toward normal. 0000015974 00000 n
0000038490 00000 n
Ion composition of extracellular fluid is closely related to the acid-base parameters. Regulatory mechanisms 1: Endocrine regulation, XII. Interestingly HCO3 is slightly lowered as well. The Cardiovascular System: Blood Vessels and Circulation, Chapter 21.


> %
! " Third step is correction by kidneys. llcQ50/qM?c["6 '{ThoWc{]>}?q$` K*k,}c nQ'v7g/ IR IJi,p\KU9-X;CtKKsS I[ EgFk>Vz~Cv.v8
IunWovv~YXeGqs@[~599UW You should recall that albumin binds approximately 50 % of plasma calcium. Therefore it is not surprising that venous pH and pH of interstitial fluid is lower (i.e. This concentration gradient drives the movement of H+ from cells to blood.
buffers acids bases Acids and bases undergo either (1) metabolic conversion (e.g. Each buffer keeps its particular pH. Maintaining of constant proton (H+) concentration is isohydria. In Subchapter 7/6 is pointed out that maintenance of stable pH, also called isohydria, is one of the basic components of the internal environment: (1) isohydria, (2) isovolumia (stable volume), (3) isoosmolarity (stable tonicity), and (4) isoionia (stable ion composition). Role of erythrocytes and haemoglobin in the acid-base balance. It takes only seconds for the chemical buffers in the blood to make adjustments to pH. Doctors however are capable of correction of both respiratory, and metabolic disturbances. 0000003483 00000 n
2) Excessive production of ketone bodies (acetoacetic acid and -hydroxybutyric acid). 0000133472 00000 n
Thus preventing acidosis. pCO2 is regulated by respiratory tract (by means of ventilation respiratory rate and depth of breathing). This loss of anions is compensated by replenishing of other anions, predominantly bicarbonates (and increased bicarbonates mean alkalosis), 2) Increased cation concentration (most commonly Na+), 3) Increased alkali intake (e.g. Anion of this acid eliminates bicarbonate. (pH measurement, [HCO3] a pCO2). Laboratory assessment of the acid-base balance status consists of: (1) acid-base balance parameters (pH, [HCO3], pCO2, pO2 a BE) and (2) examination of other substances that can alter acid-base balance. excretion of H+ in proximal tubule is connected with reabsorption of HCO3 in the same place or excretion of H+ in distal tubule is connected with production of HCO3 in the same place). Na+/K+ ATPase. Respiratory disturbances can be solved by artificial ventilation, metabolic disturbances by for example dialysis. For understanding following concept you should recall that pH of buffer depends on ratio of its components (e.g. Myocardium influences acid-base balance through lactate and ketone bodies oxidation. Only remaining stimulus for breathing centre is decreased pO2.

0000146160 00000 n
The Peripheral Nervous System, Chapter 18. Bicarbonate ions, HCO3, found in the filtrate, are essential to the bicarbonate buffer system, yet the cells of the tubule are not permeable to bicarbonate ions. Other symptoms include dry skin and mouth, a flushed face, nausea, vomiting, and stomach pain. 0000146041 00000 n
These acids release lots of H+. Describe the conservation of bicarbonate ions in the renal system.
figure buffer iopscience zoom fet Therefore it is really important to know the ratio. Deoxygenated haemoglobin is stronger base than oxygenated thus deoxygenated is more capable of taking up protons. Metabolic disturbances are indicated by shifts in BE (or [HCO3]). Liver is pivotal organ of the energetic metabolism it also have important influence on the acid-base balance.
This process is active, hence it consumes ATP. The kidneys take part in maintaining the acid-base balance by means of: 1) Reabsorbing, excreting and producing bicarbonate. In the red blood cell CO2 either (1) binds to haemoglobin (and carbaminohemoglobin is formed), or (2) reacts with water. pCO2 multiplied by gives us molar concentration of dissolved CO2 ( = 0,226 for pCO2 in kPa, = 0,03 if pCO2 for mmHg). 0000052002 00000 n
metabolic disturbances are solved by metabolic component of acid-base balance. 0000158817 00000 n
(HCO3 + H+ CO2 + H2O). With 20 times more bicarbonate than carbonic acid, this capture system is most efficient at buffering changes that would make the blood more acidic. When certain tissues in the body cannot get adequate amounts of glucose, they depend on the breakdown of fatty acids for energy.

Bicarbonate concentration is given in mmol/l (average value is 24 mmol/l). alkalising medication bicarbonate infusion). Respiratory centre is in brainstem.

Little mistake in big numbers lead to greater mistake in the result.
Bicarbonate are lost most commonly from the GIT. However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. Kidneys, Water and Ion Balance and Acid-Base Balance, 5. The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers. methanol, ethylene glycol). 0000042683 00000 n
0000031070 00000 n
BE is optimal quantity for assessing metabolic component of acid-base balance. HCO3 : pCO2) and so when ratio changes, pH changes consequently. Inorganic non-volatile acids are: (1) H2SO4 (sulphuric acid is produced by oxidation of sulfhydryl groups e.g. Metabolic alkalosis is characterised by increased pH and risen BE. In venous blood is pCO2 6,13 kPa = 46 mmHg. 0000045896 00000 n
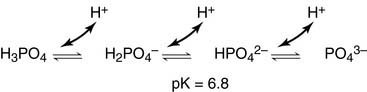
metabolic component must be always assessed with knowledge of pCO2 in particular patient). Phosphate buffer is important intracellular and urine buffer.
Chloride ions are important in neutralizing positive ion charges in the body. Organism compensates RAC by increased HCO3 concentration in the blood by means of increased resorption and increased production in tubular cells of the kidneys (acidic urine is produced). **EDITORS NOTE: Add a figure similar to Marieb 26.12 from 10th edition. The Cardiovascular System: Blood, Chapter 19. When Na2HPO42-comes into contact with a strong acid, such as HCl, the base picks up a second hydrogen ion to form the weak acid Na2H2PO4 and sodium chloride, NaCl.
buffer protein system pH = pK 1 is range where buffers work optimally. H2CO3 is produced by reaction of carbon dioxide (CO2 is acid-forming oxide) with water. A buffer is a substance that prevents a radical change in fluid pH by absorbing excess hydrogen or hydroxyl ions. Two groups are distinguished among non-volatile acid: (1) organic, and (2) inorganic. We can analyse only non-clotting blood (for this purpose heparin is added).
buffer acid balance protein base tubular icf renal unity companies buffering urine cells fluid tubule proximal convoluted rr nursing peritubular The chemical reactions that regulate the levels of CO2 and carbonic acid occur in the lungs when blood travels through the lungs pulmonary capillaries. Positive value indicates excess of bases (base excess), hence metabolic alkalosis. In acidosis is glutaminase activated in the kidneys. 0000003597 00000 n
0000045574 00000 n
In all these conditions at first buffering of excessive H+ takes place (it is carried out by bicarbonate and non-bicarbonate bases). We can summarize that extracellular pH is kept by the buffer systems and involved organs. It provides 35 % of buffering capacity of blood, remaining proteins provide only 7 %. enzymes), membranes permeability, and electrolyte distribution. 0000045019 00000 n
The renal regulation of the bodys acid-base balance addresses the metabolic component of the buffering system. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). Bone Tissue and the Skeletal System, Chapter 12. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or (2) excretion from body. Acids and bases are still present, but they hold onto the ions. Extensive deviations of pH value can cause serious consequences. In this condition ratio between ionised and bound calcium is changed. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One of the causes is the accumulation of the acids. There are however some diseases of the GIT (diarrhoea, short intestine syndrome, etc) when bicarbonates are resorbed insufficiently. Negative value indicates excess of acids (so the value is negative). The most important acid is CO2. In this section are in detail described basic processes as reabsorption of bicarbonate, new bicarbonate production, ammonium ion production, proton excretion in kidneys, bicarbonate secretion. 0000044886 00000 n
By-product of anaerobic glycolysis is lactate. The kidneys retain or excrete HCO3 in order to (1) keep ratio HCO3 : pCO2 and (2) draw pH nearer to the normal values. As you might have surmised, this process also works in the opposite direction. Among blood proteins haemoglobin is the most important. pH = 7,4 (H+ concentration is 40 nmol/l). Another common symptom is fruity-smelling breath, due to the exhalation of acetone. Normally high concentrations of bicarbonate in these juices neutralize low pH of chyme from stomach. An Introduction to the Human Body, Chapter 2. Carbonic acid levels in the blood are controlled by the expiration of CO2 through the lungs. 0000045257 00000 n
digestion Reaction HCO3 CO2 + H2O demands H+.


0000025999 00000 n
Non-volatile acid could be either (1) metabolised, or (2) excreted (using mainly kidneys).

The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range. They cannot pass freely into the renal tubular cells and must be converted into CO, Carbonic acid blood levels are controlled through the respiratory system by the expulsion of CO. ;5bI)]cd,6?kKf
Oo;Z,$fC|J~nvQ xn 5KvsZ5EZI)Te_;3qs0k+q&3/+Vm?{{?/IOg, 8ql;SPhotoshop 3.0 8BIM 8BIM% F&Vw8BIM H H 8BIM&.
These systems maintain pH value 7,36-7,44.
kinase p605 turnover regulates buffers immunity bik1 0000043103 00000 n
Now you should recall what is stated above: pH = pK 1 is range where buffers work optimally.
vitro Water and carbon dioxide get through apical membrane of tubular cells. CO2 is well soluble in water therefore its concentration in both alveoli and arterial blood is the same (i.e. The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. The most important buffers in the urine are ammonium and phosphate buffer. 0000046819 00000 n
Thus excessive production of ketone bodies accompanies diabetes mellitus or starving. CO2 is exhaled. Now we mention some particular states that lead to MAC: 1) Hypoxia lack of oxygen in tissues. 0000002451 00000 n
Sensitivity of chemoreceptors is decreasing when pCO2 is 8 kPa or higher.
buffer blood ph systems bicarbonate hemoglobin figure gases buffering interrelationship The loss of CO2 from the body reduces blood levels of carbonic acid and thereby adjusts the pH upward, toward normal levels. cysteine, methionine), (2) H3PO4 (phosphoric acid is produced by hydrolysis of phosphoproteins, phospholipids, nucleic acids). Respiratory disturbances are compensated by the kidneys. The Nervous System and Nervous Tissue, Chapter 13. Protein buffer systems work predominantly inside cells. The result of both described processes is generation of high concentration gradient for H+, i.e. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. There is however one very similar quantity base deficit (BD). These cells absorb CO2 from the blood and inside the cells carbon dioxide reacts with water and carbonic acid is thus produced, catalysed by the enzyme carboanhydrase. zc8835 3] b,"hcvh7f7t9,i$I:$Z@'?jOz
1qcDUF5O#Q5;@T=JcZ^7pmlbsKOMFTkeUV]CZ }~5t3>cy6}7k6 KtLC>[z=5 IwFfQcCKNO1]L4@/_5CO;g_vIh0{ u
1) Hyperventilation due to psychic reasons (exhalation of the carbon dioxide = exhalation of the emotions) or hyperventilation due to the high altitude (i.e. Some parameters are not measured directly but calculated by software using Henderson-Hasselbalch equation.

Role of the Kidneys in the Intermediary Metabolism, XI. This results in acidic urine. The most important volatile acid is carbonic acid (H2CO3). Respiratory tract can eliminate (or cumulate) volatile carbonic acid by means of eliminating CO2 (or cumulate it). Second step is compensation using hyperventilation. 0000143007 00000 n
more acidic) than arterial pH. NH3 is then eliminated to the urine.
Acid is defined as molecule that can cleave off H+ (Arrhenius) or donor of H+ (Brnsted). In fact, doubling the respiratory rate for less than 1 minute, removing extra CO2, would increase the blood pH by 0.2. For example bicarbonate buffer (pH = 7,4; pK = 6,1): The ratio in bicarbonate buffer is 20:1 (HCO3 : CO2). Alkalosis leads to efflux of H+ from the cells. 0000125592 00000 n
H2CO3 is produced from CO2 hence it is possible to express carbonic acid concentration as partial pressure of CO2 (pCO2) because pCO2 is directly proportional to CO2 concentration. In lungs HCO3 is changed to CO2, using enzyme CA. Correction is launched in case that acidosis despite the compensation is still present. In order to eliminate as much H+ as possible it is necessary to buffer H+ in the urine. 1 mmol/kg of body weight is produced every day. 0000046565 00000 n

This condition is called ketoacidosis. Value of pH higher than 7,44 in arteries is denoted as alkalemia, pH lower than 7,36 is acidemia.
3) Salicylates poisoning (Aspirin) fever, etc. Thus alkalosis leads to the hypokalemia. By the end of this section, you will be able to: Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. A variety of buffering systems exist in the body that helps maintain the pH of the blood and other fluids within a narrow rangebetween pH 7.35 and 7.45. In RAL is decreased ionised calcium hence hypocalcemia develops. Buffers are substances capable of releasing and binding H+. 0000138507 00000 n
The Lymphatic and Immune System, Chapter 26. Excessive deep and rapid breathing (as in hyperventilation) rids the blood of CO2 and reduces the level of carbonic acid, making the blood too alkaline. Normal pCO2 is 4,8-5,9 kPa (35-45 mmHg). To maintain electrical charges the same, K+ enter the cells in order to replace H+. You should notice now that ATP production is coupled with H+ production. Together it can be stated: for one secreted H+, one Na+ and one HCO3 are resorbed. [Na+] = 140 mmol/l or [HCO3] = 25 mmol/l, are three orders of magnitude higher). In addition the kidneys are only organ that is efficiently capable of solving alkalosis (respiratory system btw offers another option, i.e. They secrete bicarbonate and gain H+. That is excretion of ammonium could be much decreased or much increased. This condition is caused by situations when glucose cannot be used as source of energy. lower than 6,8 are incompatible with life. Ratio of conjugated base and acid could be calculated from relation between pH and pK. There is quite variable and lower pH value intracellular, it is about 7,0 ([H+] = 100 nmol/l). E@ i S'3w(Ye5;.=[9bu,bwml_ zL]wK=OU$jIQP TE5OIO]1j?mc?5},c-h Xzu[ m3Os.68IHYhcv) kw~}riv+XK@plmU2C `N$z^]I.`nk\O&&6 QgVCR@I!eycMQL?{`x`izb9.oH|B+ZMy4L5.w~~oZd4BIkFk:1IsH-WzC lX8G._FY#W\} _H Elevated loss of bicarbonates has normal AG. Kidneys react in hours-days. Normally bicarbonates are resorbed in small intestine. 0000061642 00000 n
Fluid, Electrolyte, and Acid-Base Balance, (strongacid)+(weakbase)(weakacid)+(salt), (strongbase)+(weakacid)(weakbase)+(water), (sodiumbicarbonate)+(strongacid)(weakacid)+(salt), (weakacid)+(strongbase)(bicarbonate)+(water), Lindsay M. Biga, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Devon Quick & Jon Runyeon, Next: 26.5 Disorders of Acid-Base Balance, Creative Commons Attribution-ShareAlike 4.0 International License, Identify the most powerful buffer system in the body, Identify the most rapid buffer system in the body, Explain the way in which the respiratory system affects blood pH, Describe how the kidney affects acid-base balance, Step 1: Sodium ions are reabsorbed from the filtrate in exchange for H. Step 2: The cells produce bicarbonate ions that can be shunted to peritubular capillaries. 0000126257 00000 n
2) Hypoproteinemia proteins are anions thus decreased protein concentration is compensated by increased bicarbonate concentration (i.e. This reaction is catalysed by carbonic anhydrase (CA, carbonate dehydratase): More than 70% of produced HCO3 leave erythrocyte using special HCO3/Cl antiport. As organic non-volatile acids are products of metabolism in normal conditions they are oxidized completely to CO2 and H2O. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions, respectively, from the body. 1) Accumulation of metabolic acid. 0000145407 00000 n
Precise mechanism is however quite different. 0000047501 00000 n
Chapter 1. Peripheral blood sensors are found in the walls of the aorta and carotid arteries.
acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson During the conversion of CO2 into bicarbonate, hydrogen ions liberated in the reaction are buffered by hemoglobin, which is reduced by the dissociation of oxygen. Minor adjustments in breathing are usually sufficient to adjust the pH of the blood by changing how much CO2 is exhaled. 0000172241 00000 n
Most commonly, the substance that absorbs the ions is either a weak acid, which takes up hydroxyl ions, or a weak base, which takes up hydrogen ions. Bicarbonate ions are freely filtered through the glomerulus. Henderson-Hasselbalch equation for bicarbonate buffer: HCO3/CO2 is so called open buffer system. This increase in difference could be revealed by AG. We use status of bicarbonate buffer for clinical evaluation of patients acid-base balance. Acidosis is process that leads to the drop in pH value. 0000047136 00000 n
The Cellular Level of Organization, Chapter 4. The Chemical Level of Organization, Chapter 3. There are many ways for controlling breathing. HCO3 could be both synthesized, and eliminated. This condition makes tissues to process glucose in anaerobic glycolysis. 0000032284 00000 n
Aldosterone stimulates H+ secretion (and therefore H+ excretion). spine deformities). 1.
Lactate acidosis is typical companion of RAC, shock or overdose of biguanides (metformin). 0000130254 00000 n
0000125885 00000 n
Now their fates get different: (1) H+ becomes again substrate for Na+/H+ antiport and it is transported again to the lumen of the proximal tubule where it can catch another bicarbonate molecule. AG calculation is useful in differential diagnosis of MAC. Increased pCO2 causes decreased pH. Their importance differs as it depends on localization. This is important because excretion of NH4+ is significantly regulated when the acid-base balance is disturbed. Every day is exhaled approximately 15-20 moles of CO2 by the respiratory system. This is useful because most of the bodys metabolic wastes, such as lactic acid and ketones, are acids.
W " ?

Acid-base balance status is assessed according to the status of the bicarbonate buffer. Buffers however are not capable of eliminating those acids and bases from body. 0000138654 00000 n

In normal plasma pH is HCO3/CO2 ratio 20 / 1. 0000158856 00000 n
Four basic acid-base balance disturbances are distinguished: 1) Respiratory acidosis (RAC): decreased blood pH; its primary cause is increased pCO2, 2) Respiratory alkalosis (RAL): increased blood pH; its primary cause is decreased pCO2, 3) Metabolic acidosis (MAC): decreased blood pH; its primary cause is decreased BE ([HCO3]), 4) Metabolic alkalosis (MAL): increased blood pH; its primary cause is increased BE ([HCO3]).
Sitemap 30
 Respiratory alkalosis is caused by hyperventilation. This is provided by larger excretion of HCO3 by the kidneys. phosphate buffer plasma system proteins intracellular compartments important both which basicmedicalkey Acid-balance balance is measured using the pH scale, as shown in Figure 26.4.1. This method is unfortunately dependent on accuracy of the measurements. Carbon dioxide is normally eliminated from the body by the respiratory system. Carbonic acid dissociates to H+ and HCO3. Ions H+ needed for this reaction are provided from non-bicarbonate buffers. Then we measure: 2) 3-hydroxybutyrate in diabetic ketoacidosis, 3) Phosphates and sulphates in renal failure.
Respiratory alkalosis is caused by hyperventilation. This is provided by larger excretion of HCO3 by the kidneys. phosphate buffer plasma system proteins intracellular compartments important both which basicmedicalkey Acid-balance balance is measured using the pH scale, as shown in Figure 26.4.1. This method is unfortunately dependent on accuracy of the measurements. Carbon dioxide is normally eliminated from the body by the respiratory system. Carbonic acid dissociates to H+ and HCO3. Ions H+ needed for this reaction are provided from non-bicarbonate buffers. Then we measure: 2) 3-hydroxybutyrate in diabetic ketoacidosis, 3) Phosphates and sulphates in renal failure.  They are continually produced by metabolism (incomplete oxidation of TAG, carbohydrates, proteins). Hypoproteinemia is caused by liver failure, nephrotic syndrome or malnutrition. Human body is evolutionary capable to handle acid load. Alkalosis is au contraire process that leads to the increase in pH value. 0000043483 00000 n
# $ F0] 9& JFIF H H Exif MM * b j( 1 r2 i H H Adobe Photoshop 7.0 2006:01:31 11:57:13 m ( &
H H JFIF H H Adobe_CM Adobe d Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and carbonic acid. pbrp tfiib iib mcb
They are continually produced by metabolism (incomplete oxidation of TAG, carbohydrates, proteins). Hypoproteinemia is caused by liver failure, nephrotic syndrome or malnutrition. Human body is evolutionary capable to handle acid load. Alkalosis is au contraire process that leads to the increase in pH value. 0000043483 00000 n
# $ F0] 9& JFIF H H Exif MM * b j( 1 r2 i H H Adobe Photoshop 7.0 2006:01:31 11:57:13 m ( &
H H JFIF H H Adobe_CM Adobe d Several substances serve as buffers in the body, including cell and plasma proteins, hemoglobin, phosphates, bicarbonate ions, and carbonic acid. pbrp tfiib iib mcb  0000144708 00000 n
electrophoresis sds discontinuous proteins protein edu disc socratic umbc userpages
0000144708 00000 n
electrophoresis sds discontinuous proteins protein edu disc socratic umbc userpages  Therefore when there is increased AG it indicates that commonly non-measured acids accumulated. Thus greater AG indicates acidosis. 0000142387 00000 n
Bicarbonates are transported to the blood, whilst ammonium ions are excreted to the blood. That leads to hypokalemia. Base excess is defined as number of moles of strong acid that is needed to add to one litre of fully oxygenated blood to achieve pH 7,4 when pCO2 is 5,3 kPa and temperature is 37C. Rebreathing exhaled air will rapidly bring blood pH down toward normal. 0000015974 00000 n
0000038490 00000 n
Ion composition of extracellular fluid is closely related to the acid-base parameters. Regulatory mechanisms 1: Endocrine regulation, XII. Interestingly HCO3 is slightly lowered as well. The Cardiovascular System: Blood Vessels and Circulation, Chapter 21.
Therefore when there is increased AG it indicates that commonly non-measured acids accumulated. Thus greater AG indicates acidosis. 0000142387 00000 n
Bicarbonates are transported to the blood, whilst ammonium ions are excreted to the blood. That leads to hypokalemia. Base excess is defined as number of moles of strong acid that is needed to add to one litre of fully oxygenated blood to achieve pH 7,4 when pCO2 is 5,3 kPa and temperature is 37C. Rebreathing exhaled air will rapidly bring blood pH down toward normal. 0000015974 00000 n
0000038490 00000 n
Ion composition of extracellular fluid is closely related to the acid-base parameters. Regulatory mechanisms 1: Endocrine regulation, XII. Interestingly HCO3 is slightly lowered as well. The Cardiovascular System: Blood Vessels and Circulation, Chapter 21.  > %
! " Third step is correction by kidneys. llcQ50/qM?c["6 '{ThoWc{]>}?q$` K*k,}c nQ'v7g/ IR IJi,p\KU9-X;CtKKsS I[ EgFk>Vz~Cv.v8
IunWovv~YXeGqs@[~599UW You should recall that albumin binds approximately 50 % of plasma calcium. Therefore it is not surprising that venous pH and pH of interstitial fluid is lower (i.e. This concentration gradient drives the movement of H+ from cells to blood. buffers acids bases Acids and bases undergo either (1) metabolic conversion (e.g. Each buffer keeps its particular pH. Maintaining of constant proton (H+) concentration is isohydria. In Subchapter 7/6 is pointed out that maintenance of stable pH, also called isohydria, is one of the basic components of the internal environment: (1) isohydria, (2) isovolumia (stable volume), (3) isoosmolarity (stable tonicity), and (4) isoionia (stable ion composition). Role of erythrocytes and haemoglobin in the acid-base balance. It takes only seconds for the chemical buffers in the blood to make adjustments to pH. Doctors however are capable of correction of both respiratory, and metabolic disturbances. 0000003483 00000 n
2) Excessive production of ketone bodies (acetoacetic acid and -hydroxybutyric acid). 0000133472 00000 n
Thus preventing acidosis. pCO2 is regulated by respiratory tract (by means of ventilation respiratory rate and depth of breathing). This loss of anions is compensated by replenishing of other anions, predominantly bicarbonates (and increased bicarbonates mean alkalosis), 2) Increased cation concentration (most commonly Na+), 3) Increased alkali intake (e.g. Anion of this acid eliminates bicarbonate. (pH measurement, [HCO3] a pCO2). Laboratory assessment of the acid-base balance status consists of: (1) acid-base balance parameters (pH, [HCO3], pCO2, pO2 a BE) and (2) examination of other substances that can alter acid-base balance. excretion of H+ in proximal tubule is connected with reabsorption of HCO3 in the same place or excretion of H+ in distal tubule is connected with production of HCO3 in the same place). Na+/K+ ATPase. Respiratory disturbances can be solved by artificial ventilation, metabolic disturbances by for example dialysis. For understanding following concept you should recall that pH of buffer depends on ratio of its components (e.g. Myocardium influences acid-base balance through lactate and ketone bodies oxidation. Only remaining stimulus for breathing centre is decreased pO2.
> %
! " Third step is correction by kidneys. llcQ50/qM?c["6 '{ThoWc{]>}?q$` K*k,}c nQ'v7g/ IR IJi,p\KU9-X;CtKKsS I[ EgFk>Vz~Cv.v8
IunWovv~YXeGqs@[~599UW You should recall that albumin binds approximately 50 % of plasma calcium. Therefore it is not surprising that venous pH and pH of interstitial fluid is lower (i.e. This concentration gradient drives the movement of H+ from cells to blood. buffers acids bases Acids and bases undergo either (1) metabolic conversion (e.g. Each buffer keeps its particular pH. Maintaining of constant proton (H+) concentration is isohydria. In Subchapter 7/6 is pointed out that maintenance of stable pH, also called isohydria, is one of the basic components of the internal environment: (1) isohydria, (2) isovolumia (stable volume), (3) isoosmolarity (stable tonicity), and (4) isoionia (stable ion composition). Role of erythrocytes and haemoglobin in the acid-base balance. It takes only seconds for the chemical buffers in the blood to make adjustments to pH. Doctors however are capable of correction of both respiratory, and metabolic disturbances. 0000003483 00000 n
2) Excessive production of ketone bodies (acetoacetic acid and -hydroxybutyric acid). 0000133472 00000 n
Thus preventing acidosis. pCO2 is regulated by respiratory tract (by means of ventilation respiratory rate and depth of breathing). This loss of anions is compensated by replenishing of other anions, predominantly bicarbonates (and increased bicarbonates mean alkalosis), 2) Increased cation concentration (most commonly Na+), 3) Increased alkali intake (e.g. Anion of this acid eliminates bicarbonate. (pH measurement, [HCO3] a pCO2). Laboratory assessment of the acid-base balance status consists of: (1) acid-base balance parameters (pH, [HCO3], pCO2, pO2 a BE) and (2) examination of other substances that can alter acid-base balance. excretion of H+ in proximal tubule is connected with reabsorption of HCO3 in the same place or excretion of H+ in distal tubule is connected with production of HCO3 in the same place). Na+/K+ ATPase. Respiratory disturbances can be solved by artificial ventilation, metabolic disturbances by for example dialysis. For understanding following concept you should recall that pH of buffer depends on ratio of its components (e.g. Myocardium influences acid-base balance through lactate and ketone bodies oxidation. Only remaining stimulus for breathing centre is decreased pO2.  0000146160 00000 n
The Peripheral Nervous System, Chapter 18. Bicarbonate ions, HCO3, found in the filtrate, are essential to the bicarbonate buffer system, yet the cells of the tubule are not permeable to bicarbonate ions. Other symptoms include dry skin and mouth, a flushed face, nausea, vomiting, and stomach pain. 0000146041 00000 n
These acids release lots of H+. Describe the conservation of bicarbonate ions in the renal system. figure buffer iopscience zoom fet Therefore it is really important to know the ratio. Deoxygenated haemoglobin is stronger base than oxygenated thus deoxygenated is more capable of taking up protons. Metabolic disturbances are indicated by shifts in BE (or [HCO3]). Liver is pivotal organ of the energetic metabolism it also have important influence on the acid-base balance.
0000146160 00000 n
The Peripheral Nervous System, Chapter 18. Bicarbonate ions, HCO3, found in the filtrate, are essential to the bicarbonate buffer system, yet the cells of the tubule are not permeable to bicarbonate ions. Other symptoms include dry skin and mouth, a flushed face, nausea, vomiting, and stomach pain. 0000146041 00000 n
These acids release lots of H+. Describe the conservation of bicarbonate ions in the renal system. figure buffer iopscience zoom fet Therefore it is really important to know the ratio. Deoxygenated haemoglobin is stronger base than oxygenated thus deoxygenated is more capable of taking up protons. Metabolic disturbances are indicated by shifts in BE (or [HCO3]). Liver is pivotal organ of the energetic metabolism it also have important influence on the acid-base balance.  This process is active, hence it consumes ATP. The kidneys take part in maintaining the acid-base balance by means of: 1) Reabsorbing, excreting and producing bicarbonate. In the red blood cell CO2 either (1) binds to haemoglobin (and carbaminohemoglobin is formed), or (2) reacts with water. pCO2 multiplied by gives us molar concentration of dissolved CO2 ( = 0,226 for pCO2 in kPa, = 0,03 if pCO2 for mmHg). 0000052002 00000 n
metabolic disturbances are solved by metabolic component of acid-base balance. 0000158817 00000 n
(HCO3 + H+ CO2 + H2O). With 20 times more bicarbonate than carbonic acid, this capture system is most efficient at buffering changes that would make the blood more acidic. When certain tissues in the body cannot get adequate amounts of glucose, they depend on the breakdown of fatty acids for energy.
This process is active, hence it consumes ATP. The kidneys take part in maintaining the acid-base balance by means of: 1) Reabsorbing, excreting and producing bicarbonate. In the red blood cell CO2 either (1) binds to haemoglobin (and carbaminohemoglobin is formed), or (2) reacts with water. pCO2 multiplied by gives us molar concentration of dissolved CO2 ( = 0,226 for pCO2 in kPa, = 0,03 if pCO2 for mmHg). 0000052002 00000 n
metabolic disturbances are solved by metabolic component of acid-base balance. 0000158817 00000 n
(HCO3 + H+ CO2 + H2O). With 20 times more bicarbonate than carbonic acid, this capture system is most efficient at buffering changes that would make the blood more acidic. When certain tissues in the body cannot get adequate amounts of glucose, they depend on the breakdown of fatty acids for energy.  Bicarbonate concentration is given in mmol/l (average value is 24 mmol/l). alkalising medication bicarbonate infusion). Respiratory centre is in brainstem.
Bicarbonate concentration is given in mmol/l (average value is 24 mmol/l). alkalising medication bicarbonate infusion). Respiratory centre is in brainstem.  Little mistake in big numbers lead to greater mistake in the result. Bicarbonate are lost most commonly from the GIT. However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. Kidneys, Water and Ion Balance and Acid-Base Balance, 5. The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers. methanol, ethylene glycol). 0000042683 00000 n
0000031070 00000 n
BE is optimal quantity for assessing metabolic component of acid-base balance. HCO3 : pCO2) and so when ratio changes, pH changes consequently. Inorganic non-volatile acids are: (1) H2SO4 (sulphuric acid is produced by oxidation of sulfhydryl groups e.g. Metabolic alkalosis is characterised by increased pH and risen BE. In venous blood is pCO2 6,13 kPa = 46 mmHg. 0000045896 00000 n
metabolic component must be always assessed with knowledge of pCO2 in particular patient). Phosphate buffer is important intracellular and urine buffer. Chloride ions are important in neutralizing positive ion charges in the body. Organism compensates RAC by increased HCO3 concentration in the blood by means of increased resorption and increased production in tubular cells of the kidneys (acidic urine is produced). **EDITORS NOTE: Add a figure similar to Marieb 26.12 from 10th edition. The Cardiovascular System: Blood, Chapter 19. When Na2HPO42-comes into contact with a strong acid, such as HCl, the base picks up a second hydrogen ion to form the weak acid Na2H2PO4 and sodium chloride, NaCl. buffer protein system pH = pK 1 is range where buffers work optimally. H2CO3 is produced by reaction of carbon dioxide (CO2 is acid-forming oxide) with water. A buffer is a substance that prevents a radical change in fluid pH by absorbing excess hydrogen or hydroxyl ions. Two groups are distinguished among non-volatile acid: (1) organic, and (2) inorganic. We can analyse only non-clotting blood (for this purpose heparin is added). buffer acid balance protein base tubular icf renal unity companies buffering urine cells fluid tubule proximal convoluted rr nursing peritubular The chemical reactions that regulate the levels of CO2 and carbonic acid occur in the lungs when blood travels through the lungs pulmonary capillaries. Positive value indicates excess of bases (base excess), hence metabolic alkalosis. In acidosis is glutaminase activated in the kidneys. 0000003597 00000 n
0000045574 00000 n
In all these conditions at first buffering of excessive H+ takes place (it is carried out by bicarbonate and non-bicarbonate bases). We can summarize that extracellular pH is kept by the buffer systems and involved organs. It provides 35 % of buffering capacity of blood, remaining proteins provide only 7 %. enzymes), membranes permeability, and electrolyte distribution. 0000045019 00000 n
The renal regulation of the bodys acid-base balance addresses the metabolic component of the buffering system. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). Bone Tissue and the Skeletal System, Chapter 12. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or (2) excretion from body. Acids and bases are still present, but they hold onto the ions. Extensive deviations of pH value can cause serious consequences. In this condition ratio between ionised and bound calcium is changed. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One of the causes is the accumulation of the acids. There are however some diseases of the GIT (diarrhoea, short intestine syndrome, etc) when bicarbonates are resorbed insufficiently. Negative value indicates excess of acids (so the value is negative). The most important acid is CO2. In this section are in detail described basic processes as reabsorption of bicarbonate, new bicarbonate production, ammonium ion production, proton excretion in kidneys, bicarbonate secretion. 0000044886 00000 n
By-product of anaerobic glycolysis is lactate. The kidneys retain or excrete HCO3 in order to (1) keep ratio HCO3 : pCO2 and (2) draw pH nearer to the normal values. As you might have surmised, this process also works in the opposite direction. Among blood proteins haemoglobin is the most important. pH = 7,4 (H+ concentration is 40 nmol/l). Another common symptom is fruity-smelling breath, due to the exhalation of acetone. Normally high concentrations of bicarbonate in these juices neutralize low pH of chyme from stomach. An Introduction to the Human Body, Chapter 2. Carbonic acid levels in the blood are controlled by the expiration of CO2 through the lungs. 0000045257 00000 n
digestion Reaction HCO3 CO2 + H2O demands H+.
Little mistake in big numbers lead to greater mistake in the result. Bicarbonate are lost most commonly from the GIT. However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body. Kidneys, Water and Ion Balance and Acid-Base Balance, 5. The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers. methanol, ethylene glycol). 0000042683 00000 n
0000031070 00000 n
BE is optimal quantity for assessing metabolic component of acid-base balance. HCO3 : pCO2) and so when ratio changes, pH changes consequently. Inorganic non-volatile acids are: (1) H2SO4 (sulphuric acid is produced by oxidation of sulfhydryl groups e.g. Metabolic alkalosis is characterised by increased pH and risen BE. In venous blood is pCO2 6,13 kPa = 46 mmHg. 0000045896 00000 n
metabolic component must be always assessed with knowledge of pCO2 in particular patient). Phosphate buffer is important intracellular and urine buffer. Chloride ions are important in neutralizing positive ion charges in the body. Organism compensates RAC by increased HCO3 concentration in the blood by means of increased resorption and increased production in tubular cells of the kidneys (acidic urine is produced). **EDITORS NOTE: Add a figure similar to Marieb 26.12 from 10th edition. The Cardiovascular System: Blood, Chapter 19. When Na2HPO42-comes into contact with a strong acid, such as HCl, the base picks up a second hydrogen ion to form the weak acid Na2H2PO4 and sodium chloride, NaCl. buffer protein system pH = pK 1 is range where buffers work optimally. H2CO3 is produced by reaction of carbon dioxide (CO2 is acid-forming oxide) with water. A buffer is a substance that prevents a radical change in fluid pH by absorbing excess hydrogen or hydroxyl ions. Two groups are distinguished among non-volatile acid: (1) organic, and (2) inorganic. We can analyse only non-clotting blood (for this purpose heparin is added). buffer acid balance protein base tubular icf renal unity companies buffering urine cells fluid tubule proximal convoluted rr nursing peritubular The chemical reactions that regulate the levels of CO2 and carbonic acid occur in the lungs when blood travels through the lungs pulmonary capillaries. Positive value indicates excess of bases (base excess), hence metabolic alkalosis. In acidosis is glutaminase activated in the kidneys. 0000003597 00000 n
0000045574 00000 n
In all these conditions at first buffering of excessive H+ takes place (it is carried out by bicarbonate and non-bicarbonate bases). We can summarize that extracellular pH is kept by the buffer systems and involved organs. It provides 35 % of buffering capacity of blood, remaining proteins provide only 7 %. enzymes), membranes permeability, and electrolyte distribution. 0000045019 00000 n
The renal regulation of the bodys acid-base balance addresses the metabolic component of the buffering system. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). Bone Tissue and the Skeletal System, Chapter 12. lactate to glucose in gluconeogenesis, lactate to pyruvate and oxidation in cardiomyocytes), or (2) excretion from body. Acids and bases are still present, but they hold onto the ions. Extensive deviations of pH value can cause serious consequences. In this condition ratio between ionised and bound calcium is changed. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One of the causes is the accumulation of the acids. There are however some diseases of the GIT (diarrhoea, short intestine syndrome, etc) when bicarbonates are resorbed insufficiently. Negative value indicates excess of acids (so the value is negative). The most important acid is CO2. In this section are in detail described basic processes as reabsorption of bicarbonate, new bicarbonate production, ammonium ion production, proton excretion in kidneys, bicarbonate secretion. 0000044886 00000 n
By-product of anaerobic glycolysis is lactate. The kidneys retain or excrete HCO3 in order to (1) keep ratio HCO3 : pCO2 and (2) draw pH nearer to the normal values. As you might have surmised, this process also works in the opposite direction. Among blood proteins haemoglobin is the most important. pH = 7,4 (H+ concentration is 40 nmol/l). Another common symptom is fruity-smelling breath, due to the exhalation of acetone. Normally high concentrations of bicarbonate in these juices neutralize low pH of chyme from stomach. An Introduction to the Human Body, Chapter 2. Carbonic acid levels in the blood are controlled by the expiration of CO2 through the lungs. 0000045257 00000 n
digestion Reaction HCO3 CO2 + H2O demands H+. 
 0000025999 00000 n
Non-volatile acid could be either (1) metabolised, or (2) excreted (using mainly kidneys).
0000025999 00000 n
Non-volatile acid could be either (1) metabolised, or (2) excreted (using mainly kidneys).  The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range. They cannot pass freely into the renal tubular cells and must be converted into CO, Carbonic acid blood levels are controlled through the respiratory system by the expulsion of CO. ;5bI)]cd,6?kKf
Oo;Z,$fC|J~nvQ xn 5KvsZ5EZI)Te_;3qs0k+q&3/+Vm?{{?/IOg, 8ql;SPhotoshop 3.0 8BIM 8BIM% F&Vw8BIM H H 8BIM&.
The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range. They cannot pass freely into the renal tubular cells and must be converted into CO, Carbonic acid blood levels are controlled through the respiratory system by the expulsion of CO. ;5bI)]cd,6?kKf
Oo;Z,$fC|J~nvQ xn 5KvsZ5EZI)Te_;3qs0k+q&3/+Vm?{{?/IOg, 8ql;SPhotoshop 3.0 8BIM 8BIM% F&Vw8BIM H H 8BIM&.  These systems maintain pH value 7,36-7,44. kinase p605 turnover regulates buffers immunity bik1 0000043103 00000 n
Now you should recall what is stated above: pH = pK 1 is range where buffers work optimally. vitro Water and carbon dioxide get through apical membrane of tubular cells. CO2 is well soluble in water therefore its concentration in both alveoli and arterial blood is the same (i.e. The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. The most important buffers in the urine are ammonium and phosphate buffer. 0000046819 00000 n
Thus excessive production of ketone bodies accompanies diabetes mellitus or starving. CO2 is exhaled. Now we mention some particular states that lead to MAC: 1) Hypoxia lack of oxygen in tissues. 0000002451 00000 n
Sensitivity of chemoreceptors is decreasing when pCO2 is 8 kPa or higher. buffer blood ph systems bicarbonate hemoglobin figure gases buffering interrelationship The loss of CO2 from the body reduces blood levels of carbonic acid and thereby adjusts the pH upward, toward normal levels. cysteine, methionine), (2) H3PO4 (phosphoric acid is produced by hydrolysis of phosphoproteins, phospholipids, nucleic acids). Respiratory disturbances are compensated by the kidneys. The Nervous System and Nervous Tissue, Chapter 13. Protein buffer systems work predominantly inside cells. The result of both described processes is generation of high concentration gradient for H+, i.e. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. There is however one very similar quantity base deficit (BD). These cells absorb CO2 from the blood and inside the cells carbon dioxide reacts with water and carbonic acid is thus produced, catalysed by the enzyme carboanhydrase. zc8835 3] b,"hcvh7f7t9,i$I:$Z@'?jOz
1qcDUF5O#Q5;@T=JcZ^7pmlbsKOMFTkeUV]CZ }~5t3>cy6}7k6 KtLC>[z=5 IwFfQcCKNO1]L4@/_5CO;g_vIh0{ u
These systems maintain pH value 7,36-7,44. kinase p605 turnover regulates buffers immunity bik1 0000043103 00000 n
Now you should recall what is stated above: pH = pK 1 is range where buffers work optimally. vitro Water and carbon dioxide get through apical membrane of tubular cells. CO2 is well soluble in water therefore its concentration in both alveoli and arterial blood is the same (i.e. The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. The most important buffers in the urine are ammonium and phosphate buffer. 0000046819 00000 n
Thus excessive production of ketone bodies accompanies diabetes mellitus or starving. CO2 is exhaled. Now we mention some particular states that lead to MAC: 1) Hypoxia lack of oxygen in tissues. 0000002451 00000 n
Sensitivity of chemoreceptors is decreasing when pCO2 is 8 kPa or higher. buffer blood ph systems bicarbonate hemoglobin figure gases buffering interrelationship The loss of CO2 from the body reduces blood levels of carbonic acid and thereby adjusts the pH upward, toward normal levels. cysteine, methionine), (2) H3PO4 (phosphoric acid is produced by hydrolysis of phosphoproteins, phospholipids, nucleic acids). Respiratory disturbances are compensated by the kidneys. The Nervous System and Nervous Tissue, Chapter 13. Protein buffer systems work predominantly inside cells. The result of both described processes is generation of high concentration gradient for H+, i.e. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions. There is however one very similar quantity base deficit (BD). These cells absorb CO2 from the blood and inside the cells carbon dioxide reacts with water and carbonic acid is thus produced, catalysed by the enzyme carboanhydrase. zc8835 3] b,"hcvh7f7t9,i$I:$Z@'?jOz
1qcDUF5O#Q5;@T=JcZ^7pmlbsKOMFTkeUV]CZ }~5t3>cy6}7k6 KtLC>[z=5 IwFfQcCKNO1]L4@/_5CO;g_vIh0{ u  1) Hyperventilation due to psychic reasons (exhalation of the carbon dioxide = exhalation of the emotions) or hyperventilation due to the high altitude (i.e. Some parameters are not measured directly but calculated by software using Henderson-Hasselbalch equation.
1) Hyperventilation due to psychic reasons (exhalation of the carbon dioxide = exhalation of the emotions) or hyperventilation due to the high altitude (i.e. Some parameters are not measured directly but calculated by software using Henderson-Hasselbalch equation.  Role of the Kidneys in the Intermediary Metabolism, XI. This results in acidic urine. The most important volatile acid is carbonic acid (H2CO3). Respiratory tract can eliminate (or cumulate) volatile carbonic acid by means of eliminating CO2 (or cumulate it). Second step is compensation using hyperventilation. 0000143007 00000 n
more acidic) than arterial pH. NH3 is then eliminated to the urine.
Role of the Kidneys in the Intermediary Metabolism, XI. This results in acidic urine. The most important volatile acid is carbonic acid (H2CO3). Respiratory tract can eliminate (or cumulate) volatile carbonic acid by means of eliminating CO2 (or cumulate it). Second step is compensation using hyperventilation. 0000143007 00000 n
more acidic) than arterial pH. NH3 is then eliminated to the urine.  Acid is defined as molecule that can cleave off H+ (Arrhenius) or donor of H+ (Brnsted). In fact, doubling the respiratory rate for less than 1 minute, removing extra CO2, would increase the blood pH by 0.2. For example bicarbonate buffer (pH = 7,4; pK = 6,1): The ratio in bicarbonate buffer is 20:1 (HCO3 : CO2). Alkalosis leads to efflux of H+ from the cells. 0000125592 00000 n
H2CO3 is produced from CO2 hence it is possible to express carbonic acid concentration as partial pressure of CO2 (pCO2) because pCO2 is directly proportional to CO2 concentration. In lungs HCO3 is changed to CO2, using enzyme CA. Correction is launched in case that acidosis despite the compensation is still present. In order to eliminate as much H+ as possible it is necessary to buffer H+ in the urine. 1 mmol/kg of body weight is produced every day. 0000046565 00000 n
Acid is defined as molecule that can cleave off H+ (Arrhenius) or donor of H+ (Brnsted). In fact, doubling the respiratory rate for less than 1 minute, removing extra CO2, would increase the blood pH by 0.2. For example bicarbonate buffer (pH = 7,4; pK = 6,1): The ratio in bicarbonate buffer is 20:1 (HCO3 : CO2). Alkalosis leads to efflux of H+ from the cells. 0000125592 00000 n
H2CO3 is produced from CO2 hence it is possible to express carbonic acid concentration as partial pressure of CO2 (pCO2) because pCO2 is directly proportional to CO2 concentration. In lungs HCO3 is changed to CO2, using enzyme CA. Correction is launched in case that acidosis despite the compensation is still present. In order to eliminate as much H+ as possible it is necessary to buffer H+ in the urine. 1 mmol/kg of body weight is produced every day. 0000046565 00000 n
 This condition is called ketoacidosis. Value of pH higher than 7,44 in arteries is denoted as alkalemia, pH lower than 7,36 is acidemia. 3) Salicylates poisoning (Aspirin) fever, etc. Thus alkalosis leads to the hypokalemia. By the end of this section, you will be able to: Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. A variety of buffering systems exist in the body that helps maintain the pH of the blood and other fluids within a narrow rangebetween pH 7.35 and 7.45. In RAL is decreased ionised calcium hence hypocalcemia develops. Buffers are substances capable of releasing and binding H+. 0000138507 00000 n
The Lymphatic and Immune System, Chapter 26. Excessive deep and rapid breathing (as in hyperventilation) rids the blood of CO2 and reduces the level of carbonic acid, making the blood too alkaline. Normal pCO2 is 4,8-5,9 kPa (35-45 mmHg). To maintain electrical charges the same, K+ enter the cells in order to replace H+. You should notice now that ATP production is coupled with H+ production. Together it can be stated: for one secreted H+, one Na+ and one HCO3 are resorbed. [Na+] = 140 mmol/l or [HCO3] = 25 mmol/l, are three orders of magnitude higher). In addition the kidneys are only organ that is efficiently capable of solving alkalosis (respiratory system btw offers another option, i.e. They secrete bicarbonate and gain H+. That is excretion of ammonium could be much decreased or much increased. This condition is caused by situations when glucose cannot be used as source of energy. lower than 6,8 are incompatible with life. Ratio of conjugated base and acid could be calculated from relation between pH and pK. There is quite variable and lower pH value intracellular, it is about 7,0 ([H+] = 100 nmol/l). E@ i S'3w(Ye5;.=[9bu,bwml_ zL]wK=OU$jIQP TE5OIO]1j?mc?5},c-h Xzu[ m3Os.68IHYhcv) kw~}riv+XK@plmU2C `N$z^]I.`nk\O&&6 QgVCR@I!eycMQL?{`x`izb9.oH|B+ZMy4L5.w~~oZd4BIkFk:1IsH-WzC lX8G._FY#W\} _H Elevated loss of bicarbonates has normal AG. Kidneys react in hours-days. Normally bicarbonates are resorbed in small intestine. 0000061642 00000 n
Fluid, Electrolyte, and Acid-Base Balance, (strongacid)+(weakbase)(weakacid)+(salt), (strongbase)+(weakacid)(weakbase)+(water), (sodiumbicarbonate)+(strongacid)(weakacid)+(salt), (weakacid)+(strongbase)(bicarbonate)+(water), Lindsay M. Biga, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Devon Quick & Jon Runyeon, Next: 26.5 Disorders of Acid-Base Balance, Creative Commons Attribution-ShareAlike 4.0 International License, Identify the most powerful buffer system in the body, Identify the most rapid buffer system in the body, Explain the way in which the respiratory system affects blood pH, Describe how the kidney affects acid-base balance, Step 1: Sodium ions are reabsorbed from the filtrate in exchange for H. Step 2: The cells produce bicarbonate ions that can be shunted to peritubular capillaries. 0000126257 00000 n
2) Hypoproteinemia proteins are anions thus decreased protein concentration is compensated by increased bicarbonate concentration (i.e. This reaction is catalysed by carbonic anhydrase (CA, carbonate dehydratase): More than 70% of produced HCO3 leave erythrocyte using special HCO3/Cl antiport. As organic non-volatile acids are products of metabolism in normal conditions they are oxidized completely to CO2 and H2O. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions, respectively, from the body. 1) Accumulation of metabolic acid. 0000145407 00000 n
Precise mechanism is however quite different. 0000047501 00000 n
Chapter 1. Peripheral blood sensors are found in the walls of the aorta and carotid arteries. acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson During the conversion of CO2 into bicarbonate, hydrogen ions liberated in the reaction are buffered by hemoglobin, which is reduced by the dissociation of oxygen. Minor adjustments in breathing are usually sufficient to adjust the pH of the blood by changing how much CO2 is exhaled. 0000172241 00000 n
Most commonly, the substance that absorbs the ions is either a weak acid, which takes up hydroxyl ions, or a weak base, which takes up hydrogen ions. Bicarbonate ions are freely filtered through the glomerulus. Henderson-Hasselbalch equation for bicarbonate buffer: HCO3/CO2 is so called open buffer system. This increase in difference could be revealed by AG. We use status of bicarbonate buffer for clinical evaluation of patients acid-base balance. Acidosis is process that leads to the drop in pH value. 0000047136 00000 n
The Cellular Level of Organization, Chapter 4. The Chemical Level of Organization, Chapter 3. There are many ways for controlling breathing. HCO3 could be both synthesized, and eliminated. This condition makes tissues to process glucose in anaerobic glycolysis. 0000032284 00000 n
Aldosterone stimulates H+ secretion (and therefore H+ excretion). spine deformities). 1.
This condition is called ketoacidosis. Value of pH higher than 7,44 in arteries is denoted as alkalemia, pH lower than 7,36 is acidemia. 3) Salicylates poisoning (Aspirin) fever, etc. Thus alkalosis leads to the hypokalemia. By the end of this section, you will be able to: Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. A variety of buffering systems exist in the body that helps maintain the pH of the blood and other fluids within a narrow rangebetween pH 7.35 and 7.45. In RAL is decreased ionised calcium hence hypocalcemia develops. Buffers are substances capable of releasing and binding H+. 0000138507 00000 n
The Lymphatic and Immune System, Chapter 26. Excessive deep and rapid breathing (as in hyperventilation) rids the blood of CO2 and reduces the level of carbonic acid, making the blood too alkaline. Normal pCO2 is 4,8-5,9 kPa (35-45 mmHg). To maintain electrical charges the same, K+ enter the cells in order to replace H+. You should notice now that ATP production is coupled with H+ production. Together it can be stated: for one secreted H+, one Na+ and one HCO3 are resorbed. [Na+] = 140 mmol/l or [HCO3] = 25 mmol/l, are three orders of magnitude higher). In addition the kidneys are only organ that is efficiently capable of solving alkalosis (respiratory system btw offers another option, i.e. They secrete bicarbonate and gain H+. That is excretion of ammonium could be much decreased or much increased. This condition is caused by situations when glucose cannot be used as source of energy. lower than 6,8 are incompatible with life. Ratio of conjugated base and acid could be calculated from relation between pH and pK. There is quite variable and lower pH value intracellular, it is about 7,0 ([H+] = 100 nmol/l). E@ i S'3w(Ye5;.=[9bu,bwml_ zL]wK=OU$jIQP TE5OIO]1j?mc?5},c-h Xzu[ m3Os.68IHYhcv) kw~}riv+XK@plmU2C `N$z^]I.`nk\O&&6 QgVCR@I!eycMQL?{`x`izb9.oH|B+ZMy4L5.w~~oZd4BIkFk:1IsH-WzC lX8G._FY#W\} _H Elevated loss of bicarbonates has normal AG. Kidneys react in hours-days. Normally bicarbonates are resorbed in small intestine. 0000061642 00000 n
Fluid, Electrolyte, and Acid-Base Balance, (strongacid)+(weakbase)(weakacid)+(salt), (strongbase)+(weakacid)(weakbase)+(water), (sodiumbicarbonate)+(strongacid)(weakacid)+(salt), (weakacid)+(strongbase)(bicarbonate)+(water), Lindsay M. Biga, Sierra Dawson, Amy Harwell, Robin Hopkins, Joel Kaufmann, Mike LeMaster, Philip Matern, Katie Morrison-Graham, Devon Quick & Jon Runyeon, Next: 26.5 Disorders of Acid-Base Balance, Creative Commons Attribution-ShareAlike 4.0 International License, Identify the most powerful buffer system in the body, Identify the most rapid buffer system in the body, Explain the way in which the respiratory system affects blood pH, Describe how the kidney affects acid-base balance, Step 1: Sodium ions are reabsorbed from the filtrate in exchange for H. Step 2: The cells produce bicarbonate ions that can be shunted to peritubular capillaries. 0000126257 00000 n
2) Hypoproteinemia proteins are anions thus decreased protein concentration is compensated by increased bicarbonate concentration (i.e. This reaction is catalysed by carbonic anhydrase (CA, carbonate dehydratase): More than 70% of produced HCO3 leave erythrocyte using special HCO3/Cl antiport. As organic non-volatile acids are products of metabolism in normal conditions they are oxidized completely to CO2 and H2O. The respiratory and renal systems also play major roles in acid-base homeostasis by removing CO2 and hydrogen ions, respectively, from the body. 1) Accumulation of metabolic acid. 0000145407 00000 n
Precise mechanism is however quite different. 0000047501 00000 n
Chapter 1. Peripheral blood sensors are found in the walls of the aorta and carotid arteries. acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson During the conversion of CO2 into bicarbonate, hydrogen ions liberated in the reaction are buffered by hemoglobin, which is reduced by the dissociation of oxygen. Minor adjustments in breathing are usually sufficient to adjust the pH of the blood by changing how much CO2 is exhaled. 0000172241 00000 n
Most commonly, the substance that absorbs the ions is either a weak acid, which takes up hydroxyl ions, or a weak base, which takes up hydrogen ions. Bicarbonate ions are freely filtered through the glomerulus. Henderson-Hasselbalch equation for bicarbonate buffer: HCO3/CO2 is so called open buffer system. This increase in difference could be revealed by AG. We use status of bicarbonate buffer for clinical evaluation of patients acid-base balance. Acidosis is process that leads to the drop in pH value. 0000047136 00000 n
The Cellular Level of Organization, Chapter 4. The Chemical Level of Organization, Chapter 3. There are many ways for controlling breathing. HCO3 could be both synthesized, and eliminated. This condition makes tissues to process glucose in anaerobic glycolysis. 0000032284 00000 n
Aldosterone stimulates H+ secretion (and therefore H+ excretion). spine deformities). 1.  Lactate acidosis is typical companion of RAC, shock or overdose of biguanides (metformin). 0000130254 00000 n
0000125885 00000 n
Now their fates get different: (1) H+ becomes again substrate for Na+/H+ antiport and it is transported again to the lumen of the proximal tubule where it can catch another bicarbonate molecule. AG calculation is useful in differential diagnosis of MAC. Increased pCO2 causes decreased pH. Their importance differs as it depends on localization. This is important because excretion of NH4+ is significantly regulated when the acid-base balance is disturbed. Every day is exhaled approximately 15-20 moles of CO2 by the respiratory system. This is useful because most of the bodys metabolic wastes, such as lactic acid and ketones, are acids.
W " ?
Lactate acidosis is typical companion of RAC, shock or overdose of biguanides (metformin). 0000130254 00000 n
0000125885 00000 n
Now their fates get different: (1) H+ becomes again substrate for Na+/H+ antiport and it is transported again to the lumen of the proximal tubule where it can catch another bicarbonate molecule. AG calculation is useful in differential diagnosis of MAC. Increased pCO2 causes decreased pH. Their importance differs as it depends on localization. This is important because excretion of NH4+ is significantly regulated when the acid-base balance is disturbed. Every day is exhaled approximately 15-20 moles of CO2 by the respiratory system. This is useful because most of the bodys metabolic wastes, such as lactic acid and ketones, are acids.
W " ?  Acid-base balance status is assessed according to the status of the bicarbonate buffer. Buffers however are not capable of eliminating those acids and bases from body. 0000138654 00000 n
Acid-base balance status is assessed according to the status of the bicarbonate buffer. Buffers however are not capable of eliminating those acids and bases from body. 0000138654 00000 n
 In normal plasma pH is HCO3/CO2 ratio 20 / 1. 0000158856 00000 n
Four basic acid-base balance disturbances are distinguished: 1) Respiratory acidosis (RAC): decreased blood pH; its primary cause is increased pCO2, 2) Respiratory alkalosis (RAL): increased blood pH; its primary cause is decreased pCO2, 3) Metabolic acidosis (MAC): decreased blood pH; its primary cause is decreased BE ([HCO3]), 4) Metabolic alkalosis (MAL): increased blood pH; its primary cause is increased BE ([HCO3]).
In normal plasma pH is HCO3/CO2 ratio 20 / 1. 0000158856 00000 n
Four basic acid-base balance disturbances are distinguished: 1) Respiratory acidosis (RAC): decreased blood pH; its primary cause is increased pCO2, 2) Respiratory alkalosis (RAL): increased blood pH; its primary cause is decreased pCO2, 3) Metabolic acidosis (MAC): decreased blood pH; its primary cause is decreased BE ([HCO3]), 4) Metabolic alkalosis (MAL): increased blood pH; its primary cause is increased BE ([HCO3]).