Unreacted hydrogen and nitrogen gases are then returned to the reaction vessel to undergo further reaction.
The pulverized iron is burnt (oxidized) to give magnetite or wstite (FeO, ferrous oxide) particles of a specific size. For this reason, the reduction is carried out at high gas exchange, low pressure and low temperatures. Infrared spectroscopically detected surface imides (NHad), surface amides (NH2,ad) and surface ammoniacates (NH3,ad) are formed, the latter decay under NH3 release (desorption). Experimental evidence points to reaction 2 as being the slow, rate-determining step.
ammonia The original HaberBosch reaction chambers used osmium as the catalyst, but it was available in extremely small quantities. Now lets get to the quartz. Ethics of keeping a gift card you won at a raffle at a conference your company sent you to? [3], The dissociative adsorption of nitrogen on the surface follows the following scheme, where S* symbolizes an iron atom on the surface of the catalyst:[27], The adsorption of nitrogen is similar to the chemisorption of carbon monoxide. Connect and share knowledge within a single location that is structured and easy to search. They disregard this mechanism due to experimental evidence, but lets apply some metal organic chemistry here. The energy required for this, the enthalpy H, is 206 kJ/mol.[41]. The resulting catalyst particles consist of a core of magnetite, encased in a shell of wstite, which in turn is surrounded by an outer shell of metallic iron. Uhde has developed and is using an ammonia converter with three radial flow catalyst beds and two internal heat exchangers instead of axial flow catalyst beds. [9][10] They demonstrated their process in the summer of 1909 by producing ammonia from air, drop by drop, at the rate of about 125mL (4USfloz) per hour. Unfortunately, the rapid cooling ultimately forms a catalyst of reduced abrasion resistance. The process was purchased by the German chemical company BASF, which assigned Carl Bosch the task of scaling up Haber's tabletop machine to industrial-level production. Ammonia was first manufactured using the Haber process on an industrial scale in 1913 in BASF's Oppau plant in Germany, reaching 20 tonnes per day the following year. How to run a crontab job only if a file exists?
ammonia haber process making bbc gcse science bitesize hydrogen iron catalyst nitrogen revision reaction methane steam air pressure combustion The reaction is reversible and the production of ammonia is exothermic. Cold gas is injected from the side for cooling. i How may I reduce the size of a symbol to match some other symbol? Haber initially used catalysts based on osmium and uranium.
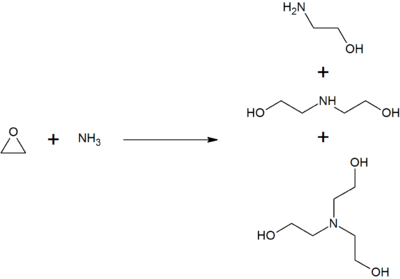
[36] The individual molecules were identified or assigned by X-ray photoelectron spectroscopy (XPS), high-resolution electron energy loss spectroscopy (HREELS) and IR spectroscopy.
monohydrate ammonia The reaction product is continuously removed for maximum yield. [3], Increased pressure does favor the forward reaction because there are 4moles of reactant for every 2moles of product, and the pressure used (1525MPa (150250bar; 2,2003,600psi)) alters the equilibrium concentrations to give a substantial ammonia yield. Nitrogen gas (N2) is very unreactive because the atoms are held together by strong triple bonds. Alternatively, the reaction mixture between the catalyst layers is cooled using heat exchangers, whereby the hydrogen-nitrogen mixture is preheated to reaction temperature. Such a process is called an absorbent-enhanced Haber process or adsorbent-enhanced Haber-Bosch process.
According to theoretical and practical studies, further improvements of the pure iron catalyst are limited.
pressure science ammonia atmospheric synthesis The process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia.
formaldehyde ammonia Possibly the reaction product in the rate-determining step is not hydrazin, but an intermediate hydrazinium which then decomposes. In order to get as much ammonia as possible in the equilibrium mixture, you need as high a pressure as possible. The surface nitride is very strongly bound to the surface.

An Eley-Rideal model would give us a rate law of the form, $$ r = k_r \; \frac{\bar{k} \: p_A^2}{(1+\bar{k} \: p_A)}$$. When flying from Preclearance airports to the US, do airlines validate your visa before letting you talk to Preclearance agents?
glucose reaction ammonia pubchem Bangalore? Haber noted uranium was almost as effective and easier to obtain than osmium. The Haber process relies on catalysts that accelerate the scission of this triple bond. [3], The reduction of the catalyst precursor magnetite to -iron is carried out directly in the production plant with synthesis gas. [42] In a subsequent distillation, the product ammonia is purified. High concentrations of hydrogen sulphide, which occur in synthesis gas from carbonization coke, are removed in a wet cleaning stage such as the Sulfosolvan process, while low concentrations are removed by adsorption on activated carbon.
NC(%3DO)O.N>>CC(C)NC(%3DO)N&format=png:w400%2Ch80%2Cb32%2C%23ffffff)
Site design / logo 2022 Stack Exchange Inc; user contributions licensed under CC BY-SA. Separately, is there an ammonia MO diagram on the web somewhere that would help to illustrate the slight bonding character of the HOMO? A comparison with vibration spectra of complex compounds allows the conclusion that the N2 molecule is bound "side-on", with an N atom in contact with a C7 site. The nitrogen in the state is more strongly bound with 31 kJmol1.
pathways ammonia formamide expected decomposition ammonia photon For low partial pressures of A, the reaction thus approximately follows first order kinetics and zeroth order for high $p_A$. The Fe(111) and Fe(211) surfaces have by far the highest activity.

This water vapor must be considered for high catalyst quality as contact with the finely divided iron would lead to premature aging of the catalyst through recrystallization, especially in conjunction with high temperatures. , {\displaystyle y_{i}}

[37] For this reason, a ratio of nitrogen to hydrogen of 1 to 3, a pressure of 250 to 350 bar, a temperature of 450 to 550C and iron are used as catalysts. 200 atmospheres is a high pressure, but not amazingly high. [36] A disadvantage of the tubular reactors was the relatively high pressure loss, which had to be applied again by compression. Was Mister Kitson and/or the planet of Kitson based on/named after George Kitson? Since the adsorption of both molecules is rapid, it cannot determine the speed of ammonia synthesis.
ammonia oxidation aerobic nitrifiers schmidt anaerobic The formation of surface nitrides makes for example chromium catalysts ineffective. is the mole fraction of the same species, {\displaystyle P^{\circ }} It only takes a minute to sign up. On each pass only about 15% conversion occurs, but any unreacted gases are recycled, and eventually an overall conversion of 97% is achieved.

Reaction 5 occurs in three steps, forming NH, NH2, and then NH3.
ammonia assay colorimetric elabscience {\displaystyle P} [46] Hydrogen atoms (Hads), which are very mobile on the catalyst surface, quickly combine with it. [3], Above this temperature, the equilibrium quickly becomes quite unfavorable for the reaction product at atmospheric pressure, according to the van 't Hoff equation. The gas mixture then still contains methane and noble gases such as argon, which, however, behave inertly.[34].
carboxylic acid ammonia reaction practice acids questions [38] The formed ammonia is continuously removed from the system. [48] The number of B5 sites depends on the size and shape of the ruthenium particles, the ruthenium precursor and the amount of ruthenium used. ^ [42], Modern ammonia reactors are designed as multi-storey reactors with low pressure drop, in which the catalysts are distributed as fills over about ten storeys one above the other. [3], Since the industrial launch of the HaberBosch process, many efforts have been made to improve it. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Furthermore, four volumetric parts of the raw materials produce two volumetric parts of ammonia. So maybe, under these conditions the bimolecular mechanism becomes the preferred pathway. Allowing milder operating pressures and temperatures, Ru-based materials are referred to as second-generation catalysts.

The mechanism of ammonia synthesis contains the following seven elementary steps: Transport and diffusion (the first and last two steps) are fast compared to adsorption, reaction and desorption because of the shell structure of the catalyst.
triethanolamine triethylamine reaction between ethanolamine ethylene oxide ammonia difference wikipedia
Sitemap 5
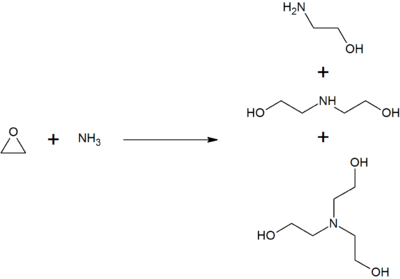 [36] The individual molecules were identified or assigned by X-ray photoelectron spectroscopy (XPS), high-resolution electron energy loss spectroscopy (HREELS) and IR spectroscopy. monohydrate ammonia The reaction product is continuously removed for maximum yield. [3], Increased pressure does favor the forward reaction because there are 4moles of reactant for every 2moles of product, and the pressure used (1525MPa (150250bar; 2,2003,600psi)) alters the equilibrium concentrations to give a substantial ammonia yield. Nitrogen gas (N2) is very unreactive because the atoms are held together by strong triple bonds. Alternatively, the reaction mixture between the catalyst layers is cooled using heat exchangers, whereby the hydrogen-nitrogen mixture is preheated to reaction temperature. Such a process is called an absorbent-enhanced Haber process or adsorbent-enhanced Haber-Bosch process.
[36] The individual molecules were identified or assigned by X-ray photoelectron spectroscopy (XPS), high-resolution electron energy loss spectroscopy (HREELS) and IR spectroscopy. monohydrate ammonia The reaction product is continuously removed for maximum yield. [3], Increased pressure does favor the forward reaction because there are 4moles of reactant for every 2moles of product, and the pressure used (1525MPa (150250bar; 2,2003,600psi)) alters the equilibrium concentrations to give a substantial ammonia yield. Nitrogen gas (N2) is very unreactive because the atoms are held together by strong triple bonds. Alternatively, the reaction mixture between the catalyst layers is cooled using heat exchangers, whereby the hydrogen-nitrogen mixture is preheated to reaction temperature. Such a process is called an absorbent-enhanced Haber process or adsorbent-enhanced Haber-Bosch process. 
 According to theoretical and practical studies, further improvements of the pure iron catalyst are limited. pressure science ammonia atmospheric synthesis The process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. formaldehyde ammonia Possibly the reaction product in the rate-determining step is not hydrazin, but an intermediate hydrazinium which then decomposes. In order to get as much ammonia as possible in the equilibrium mixture, you need as high a pressure as possible. The surface nitride is very strongly bound to the surface.
According to theoretical and practical studies, further improvements of the pure iron catalyst are limited. pressure science ammonia atmospheric synthesis The process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. formaldehyde ammonia Possibly the reaction product in the rate-determining step is not hydrazin, but an intermediate hydrazinium which then decomposes. In order to get as much ammonia as possible in the equilibrium mixture, you need as high a pressure as possible. The surface nitride is very strongly bound to the surface.  An Eley-Rideal model would give us a rate law of the form, $$ r = k_r \; \frac{\bar{k} \: p_A^2}{(1+\bar{k} \: p_A)}$$. When flying from Preclearance airports to the US, do airlines validate your visa before letting you talk to Preclearance agents? glucose reaction ammonia pubchem Bangalore? Haber noted uranium was almost as effective and easier to obtain than osmium. The Haber process relies on catalysts that accelerate the scission of this triple bond. [3], The reduction of the catalyst precursor magnetite to -iron is carried out directly in the production plant with synthesis gas. [42] In a subsequent distillation, the product ammonia is purified. High concentrations of hydrogen sulphide, which occur in synthesis gas from carbonization coke, are removed in a wet cleaning stage such as the Sulfosolvan process, while low concentrations are removed by adsorption on activated carbon.
An Eley-Rideal model would give us a rate law of the form, $$ r = k_r \; \frac{\bar{k} \: p_A^2}{(1+\bar{k} \: p_A)}$$. When flying from Preclearance airports to the US, do airlines validate your visa before letting you talk to Preclearance agents? glucose reaction ammonia pubchem Bangalore? Haber noted uranium was almost as effective and easier to obtain than osmium. The Haber process relies on catalysts that accelerate the scission of this triple bond. [3], The reduction of the catalyst precursor magnetite to -iron is carried out directly in the production plant with synthesis gas. [42] In a subsequent distillation, the product ammonia is purified. High concentrations of hydrogen sulphide, which occur in synthesis gas from carbonization coke, are removed in a wet cleaning stage such as the Sulfosolvan process, while low concentrations are removed by adsorption on activated carbon.  This water vapor must be considered for high catalyst quality as contact with the finely divided iron would lead to premature aging of the catalyst through recrystallization, especially in conjunction with high temperatures. , {\displaystyle y_{i}}
This water vapor must be considered for high catalyst quality as contact with the finely divided iron would lead to premature aging of the catalyst through recrystallization, especially in conjunction with high temperatures. , {\displaystyle y_{i}}  [37] For this reason, a ratio of nitrogen to hydrogen of 1 to 3, a pressure of 250 to 350 bar, a temperature of 450 to 550C and iron are used as catalysts. 200 atmospheres is a high pressure, but not amazingly high. [36] A disadvantage of the tubular reactors was the relatively high pressure loss, which had to be applied again by compression. Was Mister Kitson and/or the planet of Kitson based on/named after George Kitson? Since the adsorption of both molecules is rapid, it cannot determine the speed of ammonia synthesis. ammonia oxidation aerobic nitrifiers schmidt anaerobic The formation of surface nitrides makes for example chromium catalysts ineffective. is the mole fraction of the same species, {\displaystyle P^{\circ }} It only takes a minute to sign up. On each pass only about 15% conversion occurs, but any unreacted gases are recycled, and eventually an overall conversion of 97% is achieved.
[37] For this reason, a ratio of nitrogen to hydrogen of 1 to 3, a pressure of 250 to 350 bar, a temperature of 450 to 550C and iron are used as catalysts. 200 atmospheres is a high pressure, but not amazingly high. [36] A disadvantage of the tubular reactors was the relatively high pressure loss, which had to be applied again by compression. Was Mister Kitson and/or the planet of Kitson based on/named after George Kitson? Since the adsorption of both molecules is rapid, it cannot determine the speed of ammonia synthesis. ammonia oxidation aerobic nitrifiers schmidt anaerobic The formation of surface nitrides makes for example chromium catalysts ineffective. is the mole fraction of the same species, {\displaystyle P^{\circ }} It only takes a minute to sign up. On each pass only about 15% conversion occurs, but any unreacted gases are recycled, and eventually an overall conversion of 97% is achieved.  Reaction 5 occurs in three steps, forming NH, NH2, and then NH3. ammonia assay colorimetric elabscience {\displaystyle P} [46] Hydrogen atoms (Hads), which are very mobile on the catalyst surface, quickly combine with it. [3], Above this temperature, the equilibrium quickly becomes quite unfavorable for the reaction product at atmospheric pressure, according to the van 't Hoff equation. The gas mixture then still contains methane and noble gases such as argon, which, however, behave inertly.[34]. carboxylic acid ammonia reaction practice acids questions [38] The formed ammonia is continuously removed from the system. [48] The number of B5 sites depends on the size and shape of the ruthenium particles, the ruthenium precursor and the amount of ruthenium used. ^ [42], Modern ammonia reactors are designed as multi-storey reactors with low pressure drop, in which the catalysts are distributed as fills over about ten storeys one above the other. [3], Since the industrial launch of the HaberBosch process, many efforts have been made to improve it. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Furthermore, four volumetric parts of the raw materials produce two volumetric parts of ammonia. So maybe, under these conditions the bimolecular mechanism becomes the preferred pathway. Allowing milder operating pressures and temperatures, Ru-based materials are referred to as second-generation catalysts.
Reaction 5 occurs in three steps, forming NH, NH2, and then NH3. ammonia assay colorimetric elabscience {\displaystyle P} [46] Hydrogen atoms (Hads), which are very mobile on the catalyst surface, quickly combine with it. [3], Above this temperature, the equilibrium quickly becomes quite unfavorable for the reaction product at atmospheric pressure, according to the van 't Hoff equation. The gas mixture then still contains methane and noble gases such as argon, which, however, behave inertly.[34]. carboxylic acid ammonia reaction practice acids questions [38] The formed ammonia is continuously removed from the system. [48] The number of B5 sites depends on the size and shape of the ruthenium particles, the ruthenium precursor and the amount of ruthenium used. ^ [42], Modern ammonia reactors are designed as multi-storey reactors with low pressure drop, in which the catalysts are distributed as fills over about ten storeys one above the other. [3], Since the industrial launch of the HaberBosch process, many efforts have been made to improve it. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Furthermore, four volumetric parts of the raw materials produce two volumetric parts of ammonia. So maybe, under these conditions the bimolecular mechanism becomes the preferred pathway. Allowing milder operating pressures and temperatures, Ru-based materials are referred to as second-generation catalysts.  The mechanism of ammonia synthesis contains the following seven elementary steps: Transport and diffusion (the first and last two steps) are fast compared to adsorption, reaction and desorption because of the shell structure of the catalyst. triethanolamine triethylamine reaction between ethanolamine ethylene oxide ammonia difference wikipedia
The mechanism of ammonia synthesis contains the following seven elementary steps: Transport and diffusion (the first and last two steps) are fast compared to adsorption, reaction and desorption because of the shell structure of the catalyst. triethanolamine triethylamine reaction between ethanolamine ethylene oxide ammonia difference wikipedia